Blood disorders, such as beta thalassemia, Fanconi anemia, and sickle cell disease are all caused by a variation or change in a person’s genes. This change doesn’t allow blood cells and platelets to do their important job in the body. Learn how gene therapy could slow or stop disease progression for these disorders below.
Zynteglo is an FDA-approved gene therapy for adults and children with beta thalassemia who require regular red blood cell transfusions.
Casgevy is an FDA-approved gene therapy used to treat sickle cell disease and transfusion-dependent beta-thalassemia.
About Blood Disorders
Blood is very important to our body. There are different components that make up our blood, each serving a different function. Red blood cells supply oxygen to cells and tissues and also help to remove waste. White blood cells fight infectious intruders like bacteria. Platelets control blood clotting, which is vital to helping your body control a cut or an injury.
When our blood cells and platelets don't work properly it may result in severe consequences for our body. Reduced blood flow and reduced oxygen in our bodies can cause weakness, fatigue, slowed growth, severe pain and other serious complications that make it difficult to go about daily life.
Cause of Disease
A blood disorder is detected in a person by doing a blood test, such as a complete blood count (CBC). These blood disorders are inherited, meaning they are passed along from one or both biological parents. People who are considering having a child can be screened to know if they carry the gene or may meet with a genetic counselor or trusted provider to better understand any risks. If needed, find a hematologist.
Beta thalassemia is caused by a variant in the beta-globin gene, also known as the HBB gene. This gene controls how red blood cells produce hemoglobin, a protein that red blood cells need to carry oxygen. The gene variant means there is not enough hemoglobin produced for the body to function properly.
Fanconi anemia is caused by a variant in one of several genes. The disorder stops the bone marrow from making enough red blood cells, white blood cells, and platelets.
Sickle cell disease is caused by a variant in the beta-globin gene, also known as the HBB gene. This gene controls how red blood cells produce hemoglobin, a protein that red blood cells need to carry oxygen. Without enough working hemoglobin, the red blood cells become stiff and take on a sickle or crescent shape. This causes the red blood cells to break apart easily, which leads to inflammation and damage to the blood vessels. Learn more about gene therapy for sickle cell disease.
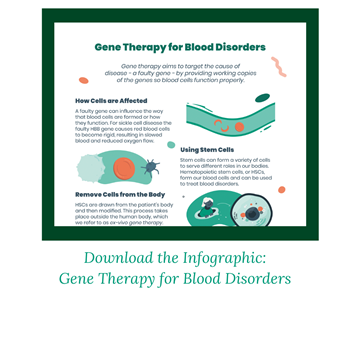 Goal of Gene Therapy
Goal of Gene Therapy
Gene therapy aims to be given one time with the goal of eliminating the need for recurring treatments, such as blood transfusions, or the need for a donor. Unlike a bone marrow transplant, a patient’s own cells are used.
This is done using an ex-vivo gene therapy approach. This means cells are removed from the person’s body, modified with new genetic instructions, and then returned to their body to begin producing healthy blood cells.
It is hematopoietic (blood) stem cells, or HSCs, that are removed from the body. This can be done through a blood draw. HSCs are versatile cells that can turn into any type of blood cell the body needs. The gene with its new instructions is delivered to the cells using a vector. Vectors are often derived from viruses because viruses have evolved to be good at getting into cells. But the viral genes are removed so only therapeutic (good) genes are delivered. These modified cells are then returned to the person’s body.
Chemotherapy is administered before gene therapy, as it eliminates existing stem cells that are still carrying the faulty gene.
In one approach, referred to as gene addition, a lentiviral vector delivers a working HHB gene to the cells. The HSCs are now instructed to produce more healthy adult hemoglobin at levels that may eliminate or reduce the need for regular blood transfusions.
Treatment Pipeline
The current standard of care for blood disorders aims to relieve symptoms, but does not alter or stop how the disease may progress. Better treatment options for people living with these diseases, such as gene therapy, are currently being investigated in clinic trials and preclinical studies. Clinical trials are a required part of the research process that help scientists understand the way a drug or treatment will interact with the human body and whether it is safe and effective. Preclinical studies are an even earlier stage of research that test the safety and effectiveness of a treatment in animal or cell-based models before proceeding with a human clinical trial.
Clinical trials may differ on various aspects of their design. If you are considering an investigational gene therapy through a clinical trial, it would be best to discuss your options with a healthcare provider or a member of the clinical trial research team.
To stay up to date on open clinical trials in the U.S. or globally, visit the ASGCT Clinical Trials Finder and search using the "diagnosis" filter.
Participating in a Clinical Trial
It is important to be well informed when deciding to participate in a clinical trial. Below are some key points to consider. Visit the considering a clinical trial page for more information and resources to help guide you.
-
Eligibility for a trial is based on strict inclusion and exclusion criteria. These are specific factors that determine whether a person can or cannot enroll in a clinical trial. This is an important way for researchers to understand if the gene therapy is working properly and to ensure participant safety. These criteria may include factors such as age, how advanced the disease is, medical history, or previous use of investigational treatments. Speak with a healthcare provider or a member of the clinical trial research team to help determine if you or your child may be eligible for a clinical trial.
-
As with any medical intervention, there are risks that need to be carefully considered. Before participating in a clinical trial, a member of the research team should review any potential risks and benefits with the patient or caregiver. Therapies being studied in clinical trials are not a guaranteed cure and cannot guarantee beneficial results. There is always a chance that the investigational treatment may not work. In the event a person is not satisfied with the outcome, the person may not receive another dose of the gene therapy. In addition, participating in a clinical trial may prevent future participation in other trials or from receiving other types of treatments. Gene therapy can be an alteration for the lifetime, so people should be aware that there could be long term effects (both good or bad) that are not known at this time.
-
Participating in a trial may offer many potential benefits compared to not receiving any form of intervention for a fatal disease. Gene therapy aims to be a one-time treatment with lasting positive effects that slow or stop disease progression for a lifetime. However, there is no guarantee. If gene therapy is received earlier in the course of disease, it has the potential to stop damage before it occurs.
-
It is the patient’s responsibility to comply with the long-term follow-up of a trial. The Food and Drug Administration (FDA) guidelines require the clinical trial research team to monitor safety and potential long-term effects of a gene therapy. Follow up may require in-person appointments that vary in frequency and location, or completion of mailed packets with response forms. The need for long-term data collection for a gene therapy trial can last up to 15 years—another reason to consider all outcomes and responsibilities that come with committing to a clinical trial. There are a limited number of participants in trials so a lack of attendance at follow-up appointments leads to not enough study data. This could negatively affect FDA approval of a new therapy and thereby limit access to the therapy by patients who did not participate in the clinical trial.
FDA-Approved Therapies
There is currently one FDA-Approved gene therapy for a blood disorder with many other treatments being researched in clinical trials.
-
Zynteglo is approved for adults and children with beta-thalassemia who require regular red blood cell transfusions.
-
Casgevy is approved to treat sickle cell disease and transfusion-dependent beta-thalassemia.
It is important to inform your primary medical provider or hematologist to understand if a gene therapy is the best option for you and to understand health insurance coverage, along with short- and long-term risks. For instance, gene therapy can be an alteration for the lifetime, so people should be aware that there could be long term effects (both good and bad) that are not known at this time.
Access
At this time, we do not know if or when more gene therapies will be approved by the FDA and commercially available for people living with different types of blood disorders. The overall process may take several more years, until it is deemed safe and effective by the FDA or regulatory agencies in other countries.
Stay Informed
A number of organizations provide individuals with blood disorders, and their families, with resources, research updates, and support:
Was this information helpful? If so, please feel free to share these resources!