The Promise of Durable Remission in B-cell Malignancies With CAR-T Persistence
Courtney Bricker-Anthony, PhD - April 24, 2023
New research published in Molecular Therapy – Oncolytics shows a relationship between durable remission and the persistence of CAR-T cells in patients with B-cell malignancies.
Although ~80% of patients with acute B-cell lymphoblastic leukemia (B-ALL) achieve complete remission following CD19-targeted CAR-T therapy, the median disease-free survival time is only 6-7 months. Multiple factors could contribute to the development of relapsed/refractory B-ALL (r/r B-ALL) following CD19 CAR-T therapy. Thus, a research team led by Drs. Yang Zhi, Qian Cheng, and Wang Sanbin sought to identify factors associated with durable remission in patients with r/r B-ALL post-CD19 CAR-T therapy in their new study published in ASGCT's open-access journal Molecular Therapy – Oncolytics.
There are two subgroups of patients who experience relapse after CD19 CAR-T therapy, namely, those with CD19 expression and those with no/weak CD19 expression. The authors previously observed that most patients who experience CD19 CAR-T loss shortly after treatment go on to experience relapse with cells expressing CD19, indicating that CAR-T persistence may be associated with durable remission. To identify factors associated with durable remission following CAR-T therapy, the authors analyzed a cohort of 50 patients with r/r B-ALL who were treated with CD19-targeted CAR-T or CD19/CD22 dual-targeted CAR-T.
Median event-free survival was similar between 35 patients treated with CD19 CAR-T (8 months) and 15 patients treated with CD19/CD22 CAR-T (7.6 months). Defining CAR-T persistence as peripheral blood CAR gene copy number ≥ 100 copies/µg genome DNA with a short-term threshold of 3 months and a long-term threshold of 6 months, the authors examined CAR-T persistence in the 35 patients who received CD19 CAR-T. After the exclusion of two patients who were followed for less than 3 months, the authors found that 13 patients treated with CD19 CAR-T achieved ≥3 months CAR-T persistence and an event-free survival rate of 58% at 24 months, and 11 of these patients achieved ≥ 6 months CAR-T persistence and 70% event-free survival at 24 months. By contrast, 20 patients who did not acquire ≥3 months CAR-T persistence had a 13% event-free survival rate at 24 months, and 22 patients who did not acquire ≥6 months CAR-T persistence had an event-free survival rate of 12%.
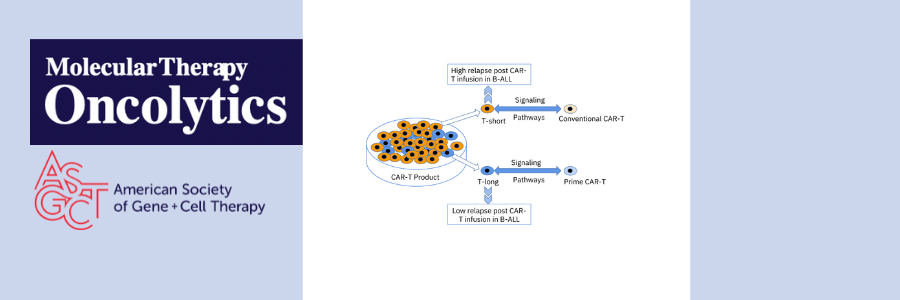
Using single cell analysis of CAR-T products used to treat two patients (one with less than 3 months of CAR-T persistence and 1 with greater than 6 months CAR-T persistence), the authors narrowed down 12 T cell subsets down to 2 T cell subsets associated with long-term persistence of CAR-T. They then combined the 2 T cell subsets into one group called T-long and combined the remaining 10 subsets into one group called T-other. Gene set variation analysis showed that T-long was more active in stem cell-related signaling and energy metabolism than T-other, suggesting the importance of early-lineage T cells in CAR-T persistence. Based on previous research suggesting that reducing the amount of time CAR-T cells spend in ex vivo culture can improve potency and stemness, the authors compared CAR-T cells that underwent 1, 2, 3, 7, or 10 days of ex vivo expansion and different activation strength. Using this information, the authors created a new CAR-T production platform, PrimeCARTM, with a shortened in vitro expansion time (~2 days) and mild activation to produce highly potent CAR-T cells with a stemness phenotype.
Next, the authors used PrimeCARTM to produce CD19-targeted or CD19/CD22 dual-targeted CAR-T cells for 8 patients with B-cell lymphoma or B-ALL. Following treatment, all eight patients achieved complete remission, with only one patient experiencing loss of CAR-T cells. At 180 days post-treatment, overall CAR-T persistence was 80%. Gene set variation analysis of conventional CAR-T cells and PrimeCAR-T cells revealed similarities between PrimeCAR-T cells and the previously identified T-long subset. Indeed, Pearson analysis demonstrated a strong positive correlation between PrimeCAR-T cell signaling pathways and T-long signaling pathways.
Overall, these findings are promising for the prevention of relapse following CAR-T cell therapy. However, some questions remain. In the initial cohort of patients with r/r B-ALL who were treated with conventional CAR-T cells, univariate risk analysis showed no significant relationship between hematopoietic stem cell transplant (HSCT) before CAR-T therapy and long-term disease-free survival. Moreover, only 2 patients who were treated with PrimeCAR-T and achieved complete remission also received HSCT. This raises the important question of whether HSCT is truly necessary to achieve durable remission of B-cell malignancies with CAR-T therapy. It is also unclear why CD19/CD22 dual-targeted CAR-T cells show less long-term persistence in vivo than their CD19 CAR-T counterparts. Additional research will be needed to address these questions and to test the efficacy of this approach in larger cohorts.
Read the full paper in Molecular Therapy – Oncolytics
Shiqi, L. et al. (2023). Durable Remission Related with CAR-T Persistence in R/R B-ALL and Long-term Persistence Potential of Prime CAR-T. Mol. Ther. Oncolytics. https://doi.org/10.1016/j.omto.2023.04.003
Courtney Bricker-Anthony, PhD, is ASGCT's scientific editor.
Related Articles