The Vector
Volume 7, Issue 7: August 2018
Editorial Team
Phillip Doerfler, PhD - Editor, The Vector
Melvin Rincon, MD, PhD - Associate Editor, The Vector
Edith Pfister, PhD - Junior Editor, The Vector
Inside This Issue
President's Message
Breaking Through
Society News
Public Policy
Industry News

President's Message
 Dear Colleagues,
Dear Colleagues,
I hope you are enjoying these last weeks of summer. At ASGCT, we are eagerly moving into our 2018-2019 programmatic year and looking forward to the 22nd Annual Meeting in Washington, DC. I am thrilled to announce that the George Stamatoyannopoulos Lecture will be given by Michel Sadelain, MD, PhD, and the Presidential Symposium lecture will be delivered by George M. Church, PhD. We greatly look forward to the comments of these thought leaders:
Michel Sadelain, MD, PhD, is the Director of the Center for Cell Engineering and the incumbent of the Stephen and Barbara Friedman Chair at Memorial Sloan-Kettering Cancer Center in New York. Dr. Sadelain’s research focuses on human cell engineering and cell therapy to treat hereditary blood disorders and cancer. His laboratory has made seminal contributions in these areas. In the field of chimeric antigen receptors, Dr. Sadelain’s lab has been involved from their conceptualization and optimization to their clinical translation for cancer immunotherapy. He previously served on the NIH Recombinant DNA Advisory Committee and is a past President of ASGCT.
George M. Church, PhD, is Robert Winthrop Professor of Genetics at Harvard Medical School in Boston, a founding member of the Wyss Institute, and initiated the Personal Genome Project, the world’s only open-access human genome and trait data sets. Dr. Church developed methods for the first human genome sequence and dramatic cost reductions since then (down from $3 billion to $600), contributing to nearly all “next generation” sequencing methods and companies. His team helped develop the use of CRISPR/Cas9 for human stem cell genome editing, as well as numerous synthetic biology technologies and applications.
Several months ago, the Trainee Committee began work on developing an anti-harassment policy for the Society. I would like to thank this committee for recognizing the need for this policy and continuing to work toward its completion. Additional thanks to the Ethics Committee and Board of Directors for their careful review of the language. Please take a moment to read the new policy.
Last month the FDA released six guidance documents on gene therapy. All six FDA guidance documents are open for comment on Regulations.gov through October 10, 2018. Reflecting steady advances in the field, three of the guidances are for disease-specific applications of gene therapy—for hemophilia, retinal disorders, and other rare diseases. The information is intended to assist sponsors in addressing the challenges of rare disease clinical development, such as limited study population size.
The other three guidance documents, which provide sponsors with manufacturing recommendations, are updates on previous guidance documents in need of revision due to advances in the field since their original release. These documents offer guidelines to sponsors regarding the provision of sufficient CMC information to assure safety, identity, quality, purity and strength/potency of gene therapy products; the proper testing for replication competent retrovirus during the manufacture of retroviral vector-based gene therapy products; and the design of long-term follow-up observational studies for the collection of data on delayed adverse events following administration of a gene therapy product.
ASGCT will be evaluating the specific content of the guidance documents over the course of the comment period and leading up to its liaison meeting with the FDA on September 13 and will provide our Society recommendations related to the details of these guidance documents. If you would like to provide input for the Society comments, please contact Betsy Foss-Campbell, Strategic Alliance Manager.
Sincerely,
Michele Calos, PhD

Breaking Through
MicroRNA-218-5p Promotes Endovascular Trophoblast Differentiation and Spiral Artery Remodeling.
Brkic, Jelena. et al.
Molecular Therapy, Published Online: July 12, 2018 doi: https://doi.org/10.1101/374785
Summary by: Jelena Brkic1
1Department of Biology, York University, Toronto, ON, Canada e-mail: brkicj@yorku.ca
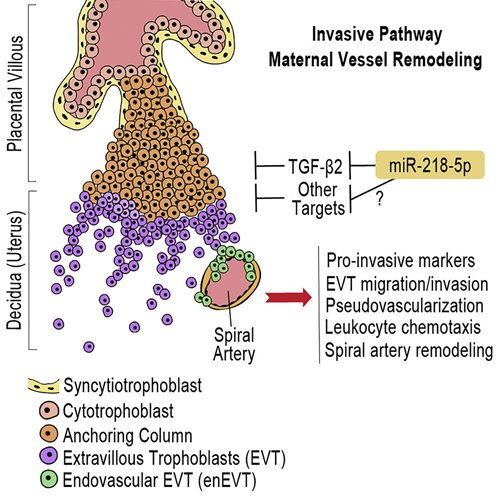 The Great Obstetrical Syndromes are associated with disorders of deep placentation and placental vascular bed abnormalities, collectively affecting 15% of pregnancies worldwide1,2. The greatest proportion is attributed to preeclampsia (PE), the leading and direct cause of maternal/neonatal morbidity and mortality3. Despite great effort, the diagnosis of PE is currently limited to late second trimester at the onset of clinical maternal symptoms of hypertension and proteinuria. Currently the only treatment for PE is delivery of placenta and baby.
The Great Obstetrical Syndromes are associated with disorders of deep placentation and placental vascular bed abnormalities, collectively affecting 15% of pregnancies worldwide1,2. The greatest proportion is attributed to preeclampsia (PE), the leading and direct cause of maternal/neonatal morbidity and mortality3. Despite great effort, the diagnosis of PE is currently limited to late second trimester at the onset of clinical maternal symptoms of hypertension and proteinuria. Currently the only treatment for PE is delivery of placenta and baby.
The pathogenesis of PE is not well understood, however, it is recognized that the placenta is the origin. A successful pregnancy depends on the health and functionality of the placenta, which requires the precise and coordinated differentiation of cytotrophoblast cells (CTBs) into the interstitial extravillous trophoblasts (iEVTs) that invade the decidua stroma, reaching as far as the inner third of the myometrium. A subset of EVTs further differentiates into endovascular EVTs (enEVTs) that invade the lumens of the maternal uterine spiral arteries during vascular transformation. Remodeling of the uterine spiral arteries up to the myometrial segments into large dilated sinusoids is essential for the adequate perfusion of the placenta to support and sustain the increasing requirements of the growing fetus4. The molecular mechanisms governing the development of these two distinct EVT subtypes are critical for elucidating the etiology of PE5.
microRNAs (miRNA) have been identified in the placenta and reported to regulate trophoblast activities6; however, their role in enEVT differentiation and spiral artery remodeling is unknown7. In this study, we investigated the function and mechanism of miR-218-5p in enEVT differentiation and vascular transformation. We report that the expression of miR-218-5p is greatly upregulated in the EVT population at time of deep invasion and enEVT differentiation. Using both established cell lines and human placental explants, we demonstrate that miR-218-5p promotes EVT migration, invasion, and enEVT differentiation.
Investigation of the mechanisms involved in spiral artery remodelling has proven difficult. This is a temporally regulated process with a complex interplay of maternal and fetal factors involving the priming of the vessels by decidual immune cells and the subsequent infiltration of enEVTs8. While studies in other hemochorial placenta species, such as in vivo rodent models, have provided many insights into the mechanism of spiral artery remodeling, their suitability as a model for humans remains under debate9. In this study, we are first to demonstrate the experimental manipulation of an ex vivo model of human placenta-decidua co-culture whereby we directly tested the ability of miR-218-5p on spiral artery remodeling. Placental explants pre-treated with miR-218-5p accelerated the decidual co-culture spiral artery remodeling process as compared to control and induced expression of endovascular markers in enEVTs in the vascular lumens. Our results suggest that miR-218-5p action may upregulate genes involved in the epithelial-to-endovascular EVT switch resulting in an increased pool of enEVT available for remodeling. The second mechanism responsible for the acceleration of spiral artery remodeling by miR-218-5p may lie in the upregulation of immune-responsive chemokines. Crosstalk between trophoblasts and the decidualized uterus is vital to the establishment of a healthy fetal-maternal interface. Closer examination of our miR-218-5p co-culture tissues shows an abundant recruitment of leukocytes along the entire length of the visible vessel compared to the control. Furthermore, miR-218-5p treated placental explants upregulated the secretion of several pro-invasive and pro-angiogenic proteins into the conditioned media. These data support a leukocyte chemotaxis effect of miR-218-5p treated trophoblasts on the surrounding maternal tissue.
The transforming growth factor (TGF)-β family is a large group of growth factors that play fundamental roles in many cellular processes, such as cellular proliferation, differentiation, apoptosis, and angiogenesis10. While the TGFβ pathway has been implicated in placental development, its role in enEVT differentiation and spiral artery remodeling has not been reported. In this study, we show that miR-218-5p targets the TGFB2 3’UTR and downregulates TGF-β2 mRNA levels and secretion. Furthermore, silencing of TGFB2 mimicked some of the miR-218-5p phenotype. Moreover, high levels of miR-218-5p expression correlated well with low TGF-β2 levels in the EVT during the most active phase of utero-placental development. These findings suggest that TGF-β2 exerts a negative effect on enEVT differentiation and its down-regulation by miR-218-5p is in part responsible for the promotion of enEVT differentiation and spiral artery remodeling by miR-218-5p.
Since miR-218-5p is downregulated in PE placenta, while serum TGF-β2 is up-regulated in PE patients11, we propose that miR-218-5p is important in normal placenta development by limiting the TGF-β2 signaling and down-regulation of miR-218-5p may contribute to the pathogenesis of PE. Shallow EVT invasion and insufficient spiral artery remodeling are shared pathophysiologies of other pregnancy complications, such as Intrauterine Growth Restriction (IUGR)12 and pre-term labor13. As such, our findings of miR-218-5p may warrant further investigation into other gestational disorders.
The search for predictive diagnostic markers has identified several potential candidates that unfortunately remain unreliable14. The use of microRNA as potential plasma biomarkers has been investigated15, and the utility of miR-218-5p as both a predictive and prognostic marker in cancer has been suggested16. The possibility of using miR-218-5p as a biomarker of PE and possibly other disorders of pregnancy remains to be investigated. Furthermore, the use of miRNA-based therapeutics is of great focus in next generation medicine17, making miR-218-5p a great candidate for exploration in early PE intervention.
1. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193-201. doi:10.1016/j.ajog.2010.08.009.
2. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21(5):521-526. doi:10.1038/ajh.2008.20.
3. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130-137. doi:10.1053/j.semperi.2009.02.010.
4. Ji L, Brkic J, Liu M, Fu G, Peng C, Wang Y-L. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol Aspects Med. 2013;34(5):981-1023. doi:10.1016/j.mam.2012.12.008.
5. Velicky P, Knöfler M, Pollheimer J. Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control. Cell Adh Migr. 2015;10(1-2):154-162. doi:10.1080/19336918.2015.1089376.
6. Hayder H, O'Brien J, Nadeem U, Peng C. MicroRNAs: crucial regulators of placental development. Reproduction. April 2018:REP–17–0603. doi:10.1530/REP-17-0603.
7. McNally R, Alqudah A, Obradovic D, McClements L. Elucidating the Pathogenesis of Pre-eclampsia Using In Vitro Models of Spiral Uterine Artery Remodelling. Curr Hypertens Rep. 2017;19(11):93. doi:10.1007/s11906-017-0786-2.
8. Harris LK. Review: Trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010;31 Suppl:S93-S98. doi:10.1016/j.placenta.2009.12.012.
9. Whitley GSJ, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31(6):465-474. doi:10.1016/j.placenta.2010.03.002.
10. Peng C. The TGF-beta superfamily and its roles in the human ovary and placenta. J Obstet Gynaecol Can. 2003;25(10):834-844.
11. Shaarawy M, Meleigy El M, Rasheed K. Maternal serum transforming growth factor beta-2 in preeclampsia and eclampsia, a potential biomarker for the assessment of disease severity and fetal outcome. J Soc Gynecol Investig. 2001;8(1):27-31.
12. Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046-1054. doi:10.1161/HYPERTENSIONAHA.113.01892.
13. Kelly R, Holzman C, Senagore P, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009;170(2):148-158. doi:10.1093/aje/kwp131.
14. Jadli A, Sharma N, Damania K, et al. Promising prognostic markers of preeclampsia: new avenues in waiting. Thromb Res. 2015;136(2):189-195. doi:10.1016/j.thromres.2015.05.011.
15. Li Q, Long A, Jiang L, et al. Quantification of preeclampsia-related microRNAs in maternal serum. Biomed Rep. 2015;3(6):792-796. doi:10.3892/br.2015.524.
16. Jiang Z, Song Q, Yang S, et al. Serum microRNA-218 is a potential biomarker for esophageal cancer. Cancer Biomark. 2015;15(4):381-389. doi:10.3233/CBM-150480.
17. Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee S-S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol Ther Nucleic Acids. 2017;8:132-143. doi:10.1016/j.omtn.2017.06.005.
.jpg)
Society News

ASGCT Releases Anti-Harassment Policy
The fields of gene and cell therapy will be strongest when all members are supported by positive, safe, and healthy work environments. To reinforce a healthy environment for all, the inaugural Trainee Committee of the American Society of Gene & Cell Therapy (ASGCT) worked together with the Executive Committee to establish a new mandatory anti-harassment and non-discrimination policy for all ASGCT events. Our hope is that this new policy will prevent incidents of harassment and discrimination, remove barriers to reporting incidents, and maintain the high quality of scientific discourse that we have come to expect at ASGCT event…read more
Read the full text of the ASGCT anti-harassment policy.

NIH NCATS is offering a funding opportunity to "support the development and evaluation of innovative approaches to deliver genome editing machinery into somatic cells." LOI due Sept. 18, applications due Oct. 18. Innovative Technologies to Deliver Genome Editing Machinery to Disease-relevant Cells and Tissues (UG3/UH3 Clinical Trial Not Allowed)
Public Policy
CMS Final Rule Insufficiently Improves Medicare Reimbursement Levels of CAR T-Cell Therapies
Last week, the Centers for Medicare and Medicaid Services (CMS) issued its final rule on the inpatient prospective payment system, which included CAR T-cell therapy reimbursement. Current Medicare reimbursement for CAR T-cell therapy leaves a significant gap in payment to hospitals compared to their combined costs for services and for the biologic, creating high risk of substantial financial losses to hospitals for providing the therapy.
ASGCT supported reimbursing the drug acquisition cost separately as a pass-through payment and assigning CAR T-cell therapy to a higher weighted diagnosis-related group (MS-DRG 016, currently used for autologous bone marrow transplant). While CMS did assign CAR T-cell therapy to the higher-weighted MS-DRG 016, it did not create pass-through payments.
CMS also approved new technology add-on payments for CAR T-cell therapies, through which Medicare reimburses a marginal cost factor of 50 percent of the costs of the new technology in excess of the DRG payment. Doing so will improve the reimbursement levels for CAR T-cell therapies from current levels, but hospitals will continue to face large gaps between their costs and reimbursement levels.
Provision Progresses on Discussing the Role of Gene Editing in National Health Security
A provision that ASGCT supported made progress last month in the House. The Senate version of the Pandemic and All-Hazards Preparedness and Advancing Innovation Act of 2018 (PAHPA) contains a provision that calls for a meeting within a year from enactment to discuss the potential role that genomic engineering technologies, including gene editing, may have in advancing national health security. An amendment containing that provision was added to the House version of the bill last month and passed out of the House Energy and Commerce Committee. The Senate version of the legislation was placed on the Senate Legislative Calendar.
ASGCT recognizes that gene editing technology has the potential to contribute to other societal benefits, in addition to therapeutic ones. The Society therefore supports discussion of the potential for additional uses of gene editing, and promotes the language in the provision that calls for inclusion of representatives from academic, private, non-profit, and other entities with expertise in genome engineering technologies, in such a meeting.
Sickle Cell Disease Legislation Moves Out of Committee
ASGCT last month signed on to a letter to Senators Lamar Alexander and Patty Murray, along with 51 other organizations in support of S. 2465, the Sickle Cell Disease Research, Surveillance, Prevention and Treatment Act of 2018.
 “This bill will allow the Department of Health and Human Services to study sickle cell disease and other heritable blood disorders, so we know how many people are affected by these conditions and implement strategies to help treat these diseases,” said Senator Lamar Alexander, chairman of the Health, Education, Labor, and Pensions (HELP) Committee. The bill passed out of the HELP Committee on July 25. A companion bill has previously passed the House.
“This bill will allow the Department of Health and Human Services to study sickle cell disease and other heritable blood disorders, so we know how many people are affected by these conditions and implement strategies to help treat these diseases,” said Senator Lamar Alexander, chairman of the Health, Education, Labor, and Pensions (HELP) Committee. The bill passed out of the HELP Committee on July 25. A companion bill has previously passed the House.
ASGCT Submits Comments to HHS on Drug Prices
Last month, ASGCT submitted a response to a request for information from the Department of Health and Human Services (HHS) regarding lowering drug prices and reducing out-of-pocket costs. In the response, the Society indicated that because the accessibility of approved gene therapies to patients is of paramount importance, it is willing to explore a variety of potential solutions to challenges to patient access: pricing, upfront costs, reimbursement, regulations, and payment models.
More specifically, ASGCT stated support for federal and state policies that sufficiently reimburse for gene therapies. The Society also encouraged further exploration of the following ways to potentially enhance patient access: value-based payment models; payment over time; a high-risk pool to limit exposure by any one payer or budget holder; and modifications to regulations that currently restrict the use of some of these solutions.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet