The Vector
Volume 9, Issue 2: February 2020
Editorial Team
Melvin Rincon, M.D., Ph.D. – Editor, The Vector
Edith Pfister, Ph.D. – Associate Editor, The Vector
Karen Bulaklak, Ph.D. – Junior Editor, The Vector
Inside This Issue
Leadership Message
Breaking Through
Society News
Career Center
Public Policy
Industry News
Leadership Message
Our Society Has Entered a New Era
 I don’t have to tell you all how much the field of gene and cell therapy has grown in the past three years. In fact, zero gene therapy products had been approved by the U.S. FDA when we last introduced a strategic plan in 2016. The Society has since entered a new era, and we have a new set of priorities that will keep pace with this ever-expanding field. I’m pleased to announce that the 2020 strategic plan, which will guide the organization for the next three years, is now online and being put into practice.
I don’t have to tell you all how much the field of gene and cell therapy has grown in the past three years. In fact, zero gene therapy products had been approved by the U.S. FDA when we last introduced a strategic plan in 2016. The Society has since entered a new era, and we have a new set of priorities that will keep pace with this ever-expanding field. I’m pleased to announce that the 2020 strategic plan, which will guide the organization for the next three years, is now online and being put into practice.
Our Board of Directors worked alongside a diverse group of ASGCT member-volunteers to divide the full plan into five priorities: communication, education, access, innovation, and global outreach. Our Strategic Planning Committee believed those five priorities represented the areas of greatest concern to our membership. I want to provide a quick rundown of some of the major objectives for each priority, but you can read more of them in the full strategic plan on our website.
The goal behind our communication priority is for ASGCT to be the most trusted source of information on the science and application of gene and cell therapy. To accomplish this, we’ll work to promote the field by delivering meaningful content and facilitate public discourse across a broad audience. ASGCT will also expand the scope of its educational content—at the Annual Meeting and during the rest of the year—by collaborating with other scientific and stakeholder organizations. To promote access, we’ll advocate on behalf of our members to lawmakers and policymakers. We’ll develop new grant and award programs to drive innovation, and we’ll engage with the National Institutes of Health to increase research funding among our members. Finally, we’ll work to expand our mission beyond the U.S. by reaching out to international professionals in the field and identifying ways to promote therapies in developing nations.
I would like to thank the Board and the Strategic Planning Committee for all of their hard work on this plan, and I look forward to seeing these efforts progress over the next three years.
Best,
Guangping Gao, Ph.D.
ASGCT President
Breaking Through
First-In-Human, First-In-Child Trial of Autologous Mesenchymal Stem Cells Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors
Ruano D, Lopez-Martin J, Moreno L, Lassaletta A, Bautista F, Andion M, Hernandez C, Gonzalez-Murillo A, Melen G, Alemany R, Madero L, Garcia-Castro J, Ramirez M
DOI: https://doi.org/10.1016/j.ymthe.2020.01.019
Summary submitted by Melvin Rincon, M.D., Ph.D.
-
This is the first clinical trial in humans testing the administration of an oncolytic virus transported by the patient's own mesenchymal cells (MSCs). Clinical development has begun in both adults and children, a population for which there are usually fewer therapeutic options.
-
The strategy is based on several intravenous administrations of the medication to reach all tumor lesions.
-
A known limitation of oncolytic virotherapy is the need for direct administration into the tumor, which poses significant challenges for the central nervous system (CNS), thoracic and abdominal cancers and limits its use to easily injectable tumors such as melanoma, sarcomas, or head and neck cancers.
-
Oncolytic viruses administered intravenously in patients with metastatic tumors encounter many physiological barriers before reaching cancer lesions. Each dose is equally threatened by the immune system. Our “Trojan horse” strategy using carrier cells seems to overcome the abovementioned limitations.
-
The toxicity found with this administration regimen has been minimal and easily tolerable by the patients. Repeated infusions of significant numbers of cells and viral particles in each patient were followed by auto limited mild or minor viral-related toxicities (i.e. fever). This point is very important in a group of patients exposed to multiple previous toxicities associated with chemotherapy and/or radiotherapy.
-
All patients recruited in the trial had recurrent/refractory metastatic solid tumor to ≥ 2 lines of conventional treatment at the time of inclusion.
-
Two pediatric patients and one adult showed disease stabilization at the end of the trial. These results confirm previously reported data of a compassionate use strategy (Melen et al. Cancer Letters 2016).
-
Using autologous MSCs imposes a 6-weeks delay in manufacturing Celyvir, a condition that may be unacceptable for patients with aggressive disease. The screening failure rate was high (> 50%), mainly because of disease progression before the medicinal product could be released from manufacturing (82%).
Oncolytic viruses are capable of becoming new medicines against cancer by destroying malignant cells. However, the administration of oncolytic viruses to patients with metastatic cancer is inefficient because the viruses do not have the ability to reach tumor lesions in significant amounts, and the patients’ immune systems are able to detect and eliminate them. We have tried a treatment with an oncolytic virus administered intravenously inside the patient's own cells for the first time in a clinical trial in children and adults with various types of advanced tumors. The results of this trial have shown that treatment is very well tolerated, patients had fever as the most frequent toxicity, but did not have other major complications. Despite being a group of patients with advanced tumors, treatment with this new medicine achieved the stabilization of the disease in two children and one adult.
Although this treatment is in its initial stages of development, our strategy of administering an oncolytic virus within carrier cells has the potential to increase the use of oncolytic virotherapy as a tool against metastatic cancer in children and adults. It allows the intravenous administration of repeated doses with very good tolerance. The limitations encountered with the use of autologous cells (from multi-treated patients with associated toxicities that may affect the quality of the starting material) can be solved using cells from healthy donors. In addition, preliminary data from our group point to intrinsic characteristics of the cells used to make the drug that is associated with clinical response. This fact opens the possibility of manipulating (genetically or pharmacologically) the cellular source to obtain an optimized version of the medicine.
Society News
The ASGCT 23rd Annual Meeting preliminary schedule is now available on the free meeting app. You can download desktop-compatible or mobile versions of the app to see live schedule updates, find speaker information, read the abstracts (starting April 28), and much more! Download the app now.
The Paying for Cures Workshop on March 10 is designed to share research and solutions to the challenge of paying for high cost, durable, and curative therapies. For more information and to register, visit this website.
The ASGCT Board has approved three new committees that are now up and running. Two of the committees—Communications and Patient Outreach—are offshoots of the former Outreach and Communications Committee. The third is the Global Outreach Committee, which will support the creation of gene therapy resources and Society programming outside of the U.S.
Career Center
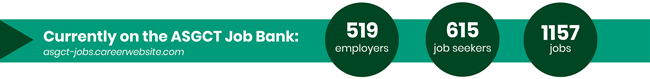
Are you looking for a job in the field of gene and cell therapy? Check out the ASGCT Job Bank and sign up to receive alerts for open jobs in your area.
If you're from a recruiting institution, advertise in the Featured Jobs section to target the 3,000+ audience of The Vector.
Featured Jobs
Public Policy
FDA Final Guidance Documents Include ASGCT Input
The FDA released six final gene therapy guidance documents on January 28. ASGCT had submitted comments on all six draft guidance documents at the end of 2018, and the FDA incorporated several of these comments into the final documents. The chair of ASGCT’s Regulatory Affairs Committee, Adora Ndu, PharmD, J.D.,applauded ASGCT's inclusion in the guidance documents in a recent blog post. Additional highlights of ASGCT-recommended changes to the final documents include that:
-
Both cells and supernatant from the vector producer cells master cell bank should be tested for replication-competent retrovirus (RCR) using a cell line selected based on the tropism of the parental virus used to generate the vector, rather than on the RCR that could potentially be generated in a given producer cell line.
-
Adverse events need not be monitored for at least five years after hemophilia treatment; rather considerations for adverse event monitoring were identified.
-
When dose-finding, placebo controls need not be added to each dose cohort for early-phase studies in subjects with rare serious or life-threatening diseases and an unmet medical need.
In addition, FDA clarified various topics for which ASGCT had requested additional information, such as that use of existing patient registries is allowable for long-term reporting.
ASGCT Advocates for Vision Research Funding
The National Eye Institute (NEI) published a request for information in December 2019 related to its upcoming five-year plan. As part of ASGCT’s recent strategic priority to engage more deeply with the NIH to assist in setting the direction of gene and cell therapy research, the Society provided comments. ASGCT emphasized the needs and gaps in gene and cell therapy research that should be addressed by NEI, including study of large animals; development of next-generation AAV capsids and non-AAV vectors; research into multigenic indications; and further preclinical study of gene editing tools in ocular contexts. Paul Sieving, M.D., Ph.D., chair of ASGCT’s Neurologic & Ophthalmic Committee and recently-retired Director of NEI, provided input on the comments along with other Committee members.
ASGCT Supports Responsible Use of Technology
ASGCT submitted feedback this month on two bills (S.252 and H.626) introduced in the Vermont legislature, both attempting to address the proliferation of unproven and unregulated stem cell clinics. The bills would require disclosure to patients that they are being administered unapproved products. ASGCT expressed support for the bills’ efforts to advance legislation to better inform patients when a provider lacks regulatory compliance for administration of products. Our comments also stressed that the state would need to be thoughtful about how it defines products subject to the bills, so as to exclude from the requirements products and procedures that do not require FDA application and approval for use.
Last week, ASGCT also submitted comments to a public consultation period on the World Health Organization’s (WHO) Draft Governance Framework for Human Genome Editing. ASGCT reiterated its position that the clinical use of germline gene editing should not currently be allowed due to the technical scientific limitations and encouraged the WHO to continue to emphasize its interim recommendation that “it would be irresponsible at this time for anyone to proceed with clinical applications of human germline genome editing.” The Society also restated that it does not currently see a need for somatic cell clinical trials to be considered part of the WHO registry, as this work is not highly distinguishable from gene therapy clinical trials and is tightly regulated around much of the world. However, should the WHO deem it necessary to include somatic cell clinical trials as part of its registry, ASGCT recommended public clarification from the WHO that its pilot registry utilizes existing data from countries with existing national registries (e.g., clinicaltrials.gov), so as to not pose additional administrative burdens on these researchers.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet