Transcribing a New Approach to Angelman Syndrome
Courtney Bricker-Anthony, PhD - February 01, 2023
Angelman syndrome is a severe neurogenetic disorder caused by the loss of UBE3A gene expression in the brain. In a recent Molecular Therapy paper, researchers showed how artificial transcription factors (ATFs) could be a promising approach to treating AS. Treated mice showed improvement in motor function, more activity in the open field test, and higher stride frequency than their untreated counterparts.
Despite the identification of causative mutations in the UBE3A gene nearly 30 years ago, Angelman syndrome remains a debilitating neurogenetic disorder that causes seizures, impaired expressive communication, sleep disturbances, and severe developmental delay.
The UBE3A gene encodes the E3 ubiquitin ligase Ube3a, which is implicated in excitatory synapse development, synaptic plasticity, and cortical development, all of which is necessary for critical functions such as learning and memory, communication, and motor coordination.
As loss of UBE3A expression contributes to the development of Angelman syndrome, gene replacement therapy seems like a good therapeutic fit for the disease. However, as explained by David J. Segal and colleagues in their recent article in Molecular Therapy, Angelman syndrome has been difficult to treat with gene therapy due to the unusual nature of UBE3A expression in the brain.
During neuronal development, the paternal copy of the UBE3A gene is imprinted in neurons but is silenced by an antisense transcript, UBE3A-ATS, which is derived from the lncRNA SNGH14. Thus, only the maternal copy of UBE3A is expressed, and mutations that alter its expression cause the disease. Nonetheless, simply restoring UBE3A expression is not as straightforward as it would seem. Overexpression of UBE3A causes 15q11-q13 duplication syndrome and is associated with autism spectrum disorders, making it necessary to tightly control UBE3A expression.
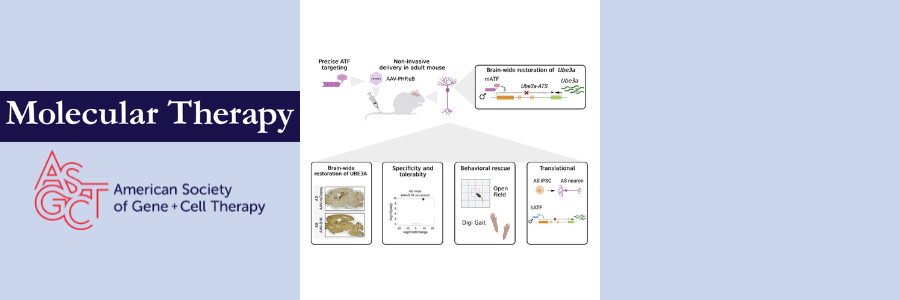
To overcome these treatment barriers, Segal and colleagues targeted Ube3a-ATS to unsilence paternal UBE3A using artificial transcription factors (ATFs) generated from modified human zinc finger proteins (ZFPs). They designed and screened 11 ZFPs in vitro to determine whether they could target the source of SNHG14: the SNURF/SNRPN locus. Their leading candidate, hATF-555, reduced SNURF expression by an impressive 87% in LNCaP cells, comparable to the performance of a CRISPR-Cas9 construct but easier to deliver due its smaller size (~0.8 kb vs. ~4.7 kb, respectively). This ATF also performed well in mature neurons differentiated from an Angelman syndrome iPSC cell line, downregulating UBE3A-ATS expression by 1.25-fold and upregulating UBE3A expression by 1.33-fold.
Next, to examine the viability of this approach in vivo, the authors treated Angelman syndrome model with mice with a previously developed ATF, ATF-S1K, that specifically targets the mouse Snurf/Snrpn locus. They packaged ATF-S1K with the AAV-PHP.eB capsid (hereafter referred to as AAV-S1K), which facilitates the passage of AAV through the blood-brain barrier following systemic delivery. The authors also added an mCherry cassette to track the AAV. Five weeks after a single injection of AAV-S1K in the tail of 6-week-old Angelman syndrome model mice, Snurf and Ube3a-ATS expression levels were reduced 2.4-fold and 2.2-fold, respectively.
In agreement with these results, UBE3A protein was detected throughout the brain of AAV-S1K-treated animals and colocalized with NeuN (neuronal marker)-positive cells, indicating restoration of Ube3a expression in neurons. AAV-S1K-treated mice also showed some improvement in motor function, exhibiting more activity in the open field test (used to evaluate general activity and exploratory behavior in mice) and higher stride frequency (a clinically relevant outcome measure for Angelman syndrome) than their untreated counterparts.
Based on these findings, targeting paternal UBE3A with artificial transcription factors may be a viable therapeutic strategy for Angelman syndrome. As with any potential therapy, questions remain regarding off-target effects, dosage, and optimal timing for treatment, which is especially relevant given the importance of intact UBE3A expression for neuronal development. Future studies will hopefully address these questions and further characterize responses to treatment in the brain, such as dendritic spine development and long-term potentiation.
Read the full open access paper now
Courtney Bricker-Anthony, PhD, is ASGCT's scientific editor.
References
1. Zampeta, F.I., Distel, B., Elgersma, Y., and Iping, R. (2022). From first report to clinical trials: a bibliometric overview and visualization of the development of Angelman syndrome research. Hum. Genet. 141, 1837–1848. 10.1007/s00439-022-02460-x.
2. Tai, H.-C., and Schuman, E.M. (2010). Angelman Syndrome: Finding the Lost Arc. Cell 140, 608–610. 10.1016/j.cell.2010.02.019.
3. O’Geen, H., Beitnere, U., Garcia, M.S., Adhikari, A., Cameron, D.L., Fenton, T.A., Copping, N.A., Deng, P., Lock, S., Halmai, J.A.N.M., et al. (2023). Transcriptional reprogramming restores UBE3A brain-wide and rescues behavioral phenotypes in an Angelman syndrome mouse model. Mol. Ther., S1525001623000138. 10.1016/j.ymthe.2023.01.013.
Related Articles