Engineering Improved Neuroprotection in Glaucoma
Courtney Bricker-Anthony, PhD - January 18, 2023
Researchers used AAV-mediated gene therapy in the eyes to enhance neuroprotection in mouse models of glaucoma—the leading cause of irreversible blindness. Intraocular injections resulted in partial recovery of visual behavior.
For decades, intraocular pressure (IOP)-lowering drugs have remained the first-line treatment for all forms of glaucoma, even normal tension glaucoma, because IOP is one of the only modifiable risk factors for glaucoma progression.
This therapeutic strategy has fallen short of its promise, with patients continuing to experience progressive, albeit slower, vision loss.
As the axons of retinal ganglion cells (RGCs) degenerate in the optic nerve, their cell bodies can remain alive for weeks but eventually succumb to apoptosis.
Loss of the RGC body is particularly devasting, as these cells have the potential for axon regeneration that will never be realized once the cell body is gone.
Thus, a team led by Takayuki Harada, MD, focused their efforts on protecting RGCs in glaucoma by promoting neurotrophin signaling, as detailed in their new article in Molecular Therapy.
Targeting neurotrophin signaling is not a novel approach. Neurotrophins such as brain-derived neurotrophic factor (BDNF) have been tested in various models of neurodegeneration with varying degrees of success.
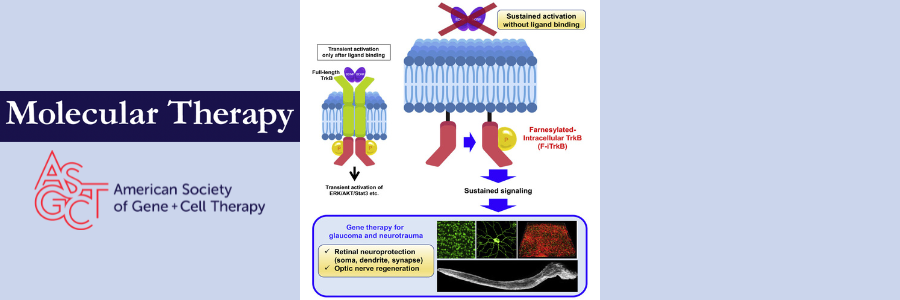
What’s unique about Harada and colleagues’ approach is how they chose to target tropomyosin receptor kinase B (TrkB). TrkB is a BDNF receptor expressed in the retina that promotes cell growth and survival. Glaucoma researchers have targeted TrkB because its expression is often downregulated in glaucoma, which limits the therapeutic effects of exogenous BDNF.
However, instead of simply restoring TrkB expression using gene therapy, Harada et al produced a constitutively active form of TrkB, bypassing the requirement for BDNF to initiate neuroprotective signaling. They obtained the modified receptor using farnesylation, a type of protein lipidation that localizes proteins at the cell membrane by covalent attachment of a lipophilic group. Farnesylation of the intracellular domain of TrkB (referred to as F-iTrkB) allowed the authors to generate an AAV2 vector with F-iTrkB and a CAG promoter to drive robust expression of the constitutively active receptor.
This unique approach reaped promising results in vitro and in vivo. Transfection of Neuro2a cells with full length TrkB (FL-TrkB) and F-iTrkB showed that both receptors are localized to the cell membrane. Western blotting revealed that F-iTrkB, even in the absence of BDNF, can induce phosphorylation of downstream signaling molecules ERK, AKT, and others at levels similar to those induced by FL-TrkB in the presence of BDNF.
Compared to empty AAV vector, intraocular injection of AAV-F-iTrkB in glutamate/aspartate transporter (GLAST) knockout mice, a model of normal tension glaucoma, preserved the thickness of the ganglion cell complex, reduced RGC loss, and improved retinal function. The authors also observed reduced RGC loss in a mouse model of ocular hypertension induced by silicone oil injection; unfortunately, silicone oil occludes the anterior chamber, which prevented assessments of visual function.
Additional promising results were obtained in mice subjected to optic nerve crush, a model of traumatic optic neuropathy that physically damages RGC axons in the optic nerve, leading to axon degeneration and RGC death. Compared to empty AAV, AAV-F-iTrkB reduced RGC loss, protected glutamatergic synapses, ameliorated dendritic arbor loss in RGCs, and preserved retinal function. Intravitreal injection of cholera toxin B (used to assess axon transport) also revealed AAV-F-iTrkB promoted axon regeneration in the optic nerve following optic nerve crush.
When the authors transected the optic nerve distal to the RGCs (just before superior colliculus), treatment with AAV-F-iTrkB partially restored visual behavior measured by optokinetics and promoted axon regeneration detected in the superior colliculus.
Based on these findings, AAV-F-iTrkB is a promising candidate for glaucoma gene therapy. Some important questions do remain, such as the long-term efficacy of AAV-F-iTrkB, the potential synergistic effects of combining AAV-F-iTrkB with other neuroprotective agents, and the use of AAV-F-iTrkB for other axonopathies (such as spinal cord injury). It would also be interesting to see how AAV-F-iTrkB performs in other models of glaucoma, such as the DBA/2J mouse or microbead occlusion model, which does not preclude assessments of visual function.
In conclusion, AAV-F-iTrkB may become a viable option for ameliorating neuronal loss and axonal degeneration in glaucoma and other neurodegenerative diseases.
The Molecular Therapy paper comes from the Tokyo Metropolitan Institute of Medical Science. Kazuhiko Namekata and ASGCT member Takayuki Harada designed the research.
Courtney Bricker-Anthony, PhD, is ASGCT's scientific editor.
Read the full paper now
References
1. Kim M, Kim DM, Park KH, Kim TW, Jeoung JW, Kim SH. Intraocular pressure reduction with topical medications and progression of normal-tension glaucoma: a 12-year mean follow-up study. Acta Ophthalmol (Copenh). 2013;91(4):e270-e275. doi:10.1111/aos.12082
2. Al-Zubidi N, Mortensen PW, Singh S. Anterograde (Wallerian) or Retrograde Degeneration in the Optic Pathway. AAO EyeWiki. Published August 2, 2022. Accessed January 11, 2022. https://eyewiki.aao.org/Anterograde_(Wallerian)_or_Retrograde_Degeneration_in_the_Optic_Pathway#cite_note-:8-11
3. Thomas CN, Berry M, Logan A, Blanch RJ, Ahmed Z. Caspases in retinal ganglion cell death and axon regeneration. Cell Death Discov. 2017;3(1):17032. doi:10.1038/cddiscovery.2017.32
4. Nishijima E, Honda S, Kitamura Y, et al. Vision protection and robust axon regeneration in glaucoma models by membrane-associated Trk receptors. Mol Ther. Published online December 2022:S1525001622006761. doi:10.1016/j.ymthe.2022.11.018
5. Lambuk L, Mohd Lazaldin MA, Ahmad S, et al. Brain-Derived Neurotrophic Factor-Mediated Neuroprotection in Glaucoma: A Review of Current State of the Art. Front Pharmacol. 2022;13:875662. doi:10.3389/fphar.2022.875662
Related Articles