The Vector
Volume 8, Issue 4: April 2019
Editorial Team
Phillip Doerfler, PhD - Editor, The Vector
Melvin Rincon, MD, PhD - Associate Editor, The Vector
Edith Pfister, PhD - Junior Editor, The Vector
Inside This Issue
Leadership Message
Breaking Through
Society News
Public Policy
Industry News
President's Message
The Future of ASGCT and the Annual Meeting

I have had the honor of serving as president of ASGCT for the past year and have spent much of that time helping to plan the 22nd Annual Meeting alongside the Program Committee, the staff, and a small army of ASGCT volunteers.
Thanks to the diligent work of all those teams and the booming interest in our field, the 22nd Annual Meeting will be the most highly attended in ASGCT’s history. In order to accommodate the increased attendance, we have expanded our on-site live streaming services, added more break-out sessions to alleviate congestion, and ensured that the exhibit hall will be open throughout the duration of the meeting.
The success of the Annual Meeting is a leading indicator of the health of our field, and I have never been more optimistic about the future of ASGCT!
The Strategic Planning workgroup met in January to develop a series of priorities and initiatives to guide the Society’s work for the next three years. The plan will be finalized this month at the 22nd Annual Meeting, and details on the direction of ASGCT’s growth and development will be announced throughout the year.
In addition to ASGCT’s current programming, the workgroup created strong new directives to support researchers, trainees, clinicians, advocates, and the public, as well as increased global outreach.
We are excited about the ideas that emerged and will be sharing the plans with the membership in the near future.
Finally, I thank the entire ASGCT community for allowing me to serve as part of the Society’s Executive Committee for the past three years and as president for 2018-19. It has been an honor and a high point of my career. I am incredibly proud of the continuing growth of ASGCT that we have witnessed during my term, and I look forward to participating in the bold future we’ve laid out for the Society!
Yours sincerely,
Michele P. Calos, Ph.D.
ASGCT President
Advertisement

Advertisement
Breaking Through
In Vivo Imaging of Oncolytic Measles Virus Propagation with Single-Cell Resolution
Iris Kemler, Matthew K. Ennis, Claudia M. Neuhauser, and David Dingli
Summary by Iris Kemler and David Dingli
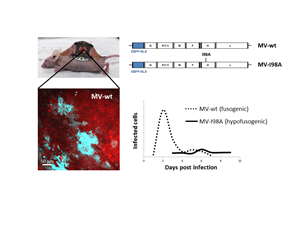
Replication competent measles viruses (MVs) have oncolytic activity against a wide variety of human cancers and are evaluated in several clinical trials. Recently it was reported that a single, systemic injection of an engineered oncolytic measles virus expressing the sodium iodide symporter gene (MV-NIS) led to a complete and durable (> 5 years) remission in a patient with disseminated, relapsed and refractory multiple myeloma. Unfortunately, other patients receiving similar treatment did not have such a favorable outcome. The reasons behind these disparate results are unclear.
Tumor virotherapy is a race between the virus, tumor and the immune system. In order to improve oncolytic virotherapy it is important to understand and document the dynamic interactions between the virus and the tumor. To this end, viruses have been engineered to express transgenes that enable us to track virus replication by various in vivo imaging techniques. However, the spatial resolution of those techniques are limited to 1-2mm for bioluminescence (luciferase) and 0.25mm for microCT/SPECT (radioactivity) and, therefore, are restricted to the macroscopic scale of organs. In contrast, multiphoton imaging with fluorophores allows for subcellular resolution and accurate quantification of the number of infected cells in a specific volume of tumor. We have combined two-photon imaging with an intravital imaging system using the dorsal skin fold chamber (DSFC), and achieve serial, non-invasive imaging in live animals over several days.
Orthotopic tumors expressing a fluorescent protein were grown dorsally in athymic nude mice. Once the tumors reached a size of about 0.4cm, the DSFC was implanted over the tumor. Subsequently a replication competent MV based on the Edmonston vaccine strain, was injected into the tumor. This virus was engineered to express the enhanced blue fluorescent protein (EBFP) containing a nuclear localization sequence (NLS). Every MV infected cell accumulates EBFP exclusively in the nucleus which clearly distinguishes one infected cell from the next one and enables easy quantification of infected tumor cells. Virus replication was imaged over several days by two-photon microscopy and the number of infected cells was quantitated with the Imaris image analysis software.
We compared the replication dynamics of a wild-type fusogenic MV (MV-EBFP-NLS) with those of a hypofusogenic MV (MV-EBFP-I98A-NLS) which has a mutation (I98A) in the fusion activation segment of the hemagglutinin gene. This virus has normal receptor binding, but infects cells at a lower rate than the wild-type virus, due to impairment in fusion triggering.
We observe that both viruses establish multiple foci of infection within the tumor and are capable of forming multinucleated syncytia. However, the replication kinetics of these two viruses are distinctly different. The fusogenic virus spreads very fast: infected cells were detected as early as 1dpi and the peak of infection occurred 2-3 days after virus administration. In contrast, the hypofusogenic virus reached the maximum number of infected tumor cells around 6 days post infection. The two viruses also differ in the size of their infection foci, with the fusogenic one comprising on average 3.6 times more infected cells per focus than the hypofusogenic virus. Interestingly, despite the presence of high-level infection, many areas within the tumor remain uninfected. Therefore it appears that barriers to virus spread remain that limit its access to areas within the tumor.
Information gained from these in vivo high-resolution imaging studies can be used to further understand and optimize MV oncolytic virotherapy. This system can be adapted to other preclinical models, using different cell lines and viruses, including immunocompetent mouse models in which the influence of the immune system on tumor oncolysis could be investigated.
Advertisement

Advertisement
Society News
Call for Applications: Junior Editor, The Vector
ASGCT is looking for a new junior editor to join The Vector's editorial team. The junior editor serves a one year term, appointed every year by the ASGCT Publications Committee. Candidates are asked to submit a CV, statement of interest, and 500 word summary of this article. At the conclusion of the junior editor's term, the successful volunteer is expected to ascend to the position of associate editor and, ultimately, editor.
The roles and responsibilities are as follows:
- Coordinate with ASGCT staff and the editors to solicit scientific content and summaries for Breaking Through section of The Vector
- Review and edit scientific summaries before they are added to The Vector
- Participate on planning conference calls with the Editors and ASGCT staff
If you are interested in serving as the junior editor or have any questions, please send your CV, a brief statement of interest, and a 500 word summary of this article to Alex Wendland, ASGCT digital communications manager.
Five Members Elected to ASGCT Board of Directors
All five incoming members of the ASGCT Board of Directors will assume their roles following the 22nd Annual Meeting, held April 29-May 2 at the Washington Hilton in Washington, D.C.
-
Vice President
Beverly Davidson, Ph.D.
Children's Hospital of Philadelphia
-
Secretary
Terence R. Flotte, M.D.
University of Massachusetts Medical School
-
At-Large Director and Membership Council Chair
Maritza McIntyre, Ph.D.
Advanced Therapy Partners, LLC
-
At-Large Director
Matthew Wilson, M.D., Ph.D.
Vanderbilt University
-
At-Large Director
Philip Gregory, D.Phil.
bluebird bio
ASGCT Members and Transitional Members who are current with their membership dues are eligible to vote in ASGCT elections.
Learn more about the new board members on ASGCT.org.
Advertisement

Advertisement
Public Policy
Recent FDA Rare Disease Guidance Includes ASGCT-Requested Info
ASGCT submitted comments this month on the FDA’s draft guidance on Rare Diseases: Common Issues in Drug Development. In the comments, the Society expressed gratitude to the Agency for the inclusion of information not present in the Human Gene Therapy for Rare Diseases draft guidance issued last year. As ASGCT recommended in comments on that gene therapy-specific guidance, the recent guidance acknowledges the limited availability of natural history for many rare diseases, clarifies the expectations for analytical validation of biomarkers, and provides additional recommendations for product development in the pediatric population. ASGCT’s recommendations for changes include identifying ways in which retrospective natural history studies may be useful; clarifying expectations for clinical validation of biomarkers; elaborating on the use of adaptive trial designs; and expounding on the flexibility that the Agency could provide on inspection planning and validation strategies.
ASGCT Supports Enhanced Medicare Reimbursement Levels
ASGCT signed onto a letter this month to the Centers for Medicare and Medicaid Services (CMS) supporting improved Medicare inpatient reimbursement levels for CAR T-cell therapies, to promote sustained patient access to these lifesaving treatments. Because current reimbursement mechanisms fail to adequately reimburse providers for variable patient care costs and the cost of the product, the letter proposes that CMS implement a policy (cost-to-charge ratio of 1.0) that would allow providers to report the actual product cost without markup. The letter also requests that CMS increase the new technology add-on payment for CAR T-cell therapies to 80 percent of the product cost (from the current 50 percent).
Annual Meeting Addresses Policy Topics
During the Annual Meeting, there will be a number of policy-related topics addressing both legislative and regulatory issues facing the field today. Below are the symposia and workshops you can attend to stay up-to-date on the issues.
Public Policy
- Post-Approval Commercialization workshop* session, Achieving Sustainable Patient and Market Access on Sunday, April 28, at 3 – 6 p.m.
- Symposium titled Pricing, Access, and Value of Gene Therapies: What Are the Real-World Challenges and Solutions? on Monday, April 29, at 8 – 10 a.m.
- Symposium titled Right to Try or Wrong to Try? on Wednesday, May 1, at 8-10 a.m.
Regulatory Policy
- Post-Approval Commercialization workshop* keynote address by Jessica Tucker, PhD, Director, Biosafety, Biosecurity, and Emerging Biotechnology at NIH, Modernizing Gene Therapy Oversight and the Role of the RAC on Sunday, April 28, at 10 - 10:30 a.m.
- Post-Approval Commercialization workshop* session, Post-Approval Change Management and Overall Impact on Commercialization on Sunday, April 28, at 1 – 2:30 p.m.
- Pre-Approval Commercialization workshop** session, Global Regulatory Update: Outlook in 2019 – Policy Approaches and Considerations in Gene Therapy on Sunday, April 28, at 1-2:30 p.m.
- Symposium titled Getting to the Finish Line: Market Success on Thursday, May 2, at 8 -10 a.m.
* This workshop requires a separate registration.You can register for the Post-Approval Commercialization workshop .
** The Pre-Approval Commercialization workshop is sold out.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet
Thank you to our platinum and gold Annual Meeting Sponsors
Platinum
.aspx?width=210&height=105)

.aspx?width=210&height=105)
.aspx?width=210&height=105)



Gold
.png)


.aspx?width=160&height=80)
.aspx?width=160&height=80)







