The Vector
Volume 8, Issue 1: January 2019
Editorial Team
Phillip Doerfler, PhD - Editor, The Vector
Melvin Rincon, MD, PhD - Associate Editor, The Vector
Edith Pfister, PhD - Junior Editor, The Vector
Inside This Issue
President's Message
Breaking Through
Society News
Public Policy
Industry News
Outreach and Communications Message
ASGCT Patient Education Program Debuts on January 17
 ASGCT is set to start the New Year on the right foot by debuting our brand new patient education program and premiering the first of our ASGCT-sponsored episodes of SciShow, an educational YouTube series, later this month.
ASGCT is set to start the New Year on the right foot by debuting our brand new patient education program and premiering the first of our ASGCT-sponsored episodes of SciShow, an educational YouTube series, later this month.
The Outreach and Communications Committee and the ASGCT staff has partnered with Demo Duck, a third-party communications group with a long history of medical education to create a patient-centered portal. ASGCT’s new patient education portal guides patients, families, and patient advocates through the basics of gene therapy, various treatment approaches, the clinical trials process, and five specific diseases on the verge of major treatment advancements thanks to gene and cell therapies.
The five diseases featured in the initial rollout of the ASGCT Patient Education program are:
- Blood disorders
- Inherited retinal disorders
- Leukodystrophy
- Spinal muscular atrophy
- X-Linked myotubular myopathy
We’re excited to share all of this brand new content with the community when the first pages debut on January 17.
Finally, in celebration of our brand new patient education materials, ASGCT has sponsored a three-episode run of SciShow, a popular educational YouTube series, focused on gene therapy. The first ASGCT-sponsored episode will debut on the SciShow channel on January 21 and will focus on gene therapy efforts to combat blood disorders, specifically sickle cell disease and beta-thalassemia.
Thank you to all of the staff, stakeholders, and ASGCT volunteers who have worked so hard to create this important content.
Happy New Year,
Cenk Sumen, Ph.D.
Chair, Outreach and Communications Committee

Breaking Through
Enhanced Delivery of Oncolytic Adenovirus by Neural Stem Cells for Treatment of Metastatic Ovarian Cancer
Rachael Mooney, Asma Abdul Majid, Jennifer Batalla-Covello, Diana Machado, Xueli Liu, Joanna Gonzaga, Revathi Tirughanaswari, Mohamed Hamed, Maciej S.Lesniak, David T.Curiel, Karen S.Aboody
Summary by Rachael Mooney and Karen S. Aboody
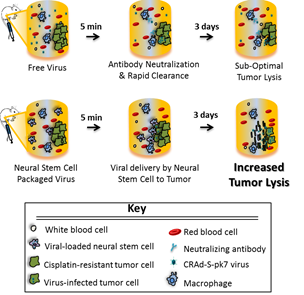 Conditionally replication-competent oncolytic virotherapy is a highly promising cancer treatment approach, irrespective of tumor radiation or drug resistance. Although favorable clinical safety profiles have been demonstrated with adenovirus subtype 5-based virotherapies, their therapeutic efficacy has been disappointing. A major hurdle limiting efficacy in patients with abdominal ovarian metastases is that the majority of free virus particles are rapidly cleared following intraperitoneal delivery due to their small size (~100 nanometers) and the presence of neutralizing antibodies. Therefore, there is suboptimal viral infection of tumor cells limiting distribution through tumor foci. Delivery hurdles for oncolytic adenoviruses are particularly high, because most patients have pre-existing immunity to these common human viruses.
Conditionally replication-competent oncolytic virotherapy is a highly promising cancer treatment approach, irrespective of tumor radiation or drug resistance. Although favorable clinical safety profiles have been demonstrated with adenovirus subtype 5-based virotherapies, their therapeutic efficacy has been disappointing. A major hurdle limiting efficacy in patients with abdominal ovarian metastases is that the majority of free virus particles are rapidly cleared following intraperitoneal delivery due to their small size (~100 nanometers) and the presence of neutralizing antibodies. Therefore, there is suboptimal viral infection of tumor cells limiting distribution through tumor foci. Delivery hurdles for oncolytic adenoviruses are particularly high, because most patients have pre-existing immunity to these common human viruses.
To overcome these limitations, there is increasing interest in developing tumor-tropic cell carriers to deliver viral agents. The ideal cell carrier for conditionally replication competent adenovirus (CRAd) delivery would be chromosomally stable, support viral infection and amplification, shield the viruses from the host immune system en route to tumors, and provide for intrinsic homing, penetration and seeding of virus to tumor metastases. Patient-derived mesenchymal stem cells have been most widely investigated for delivering virus to ovarian tumors, but high costs and variability of autologous cell preparations preclude routine clinical use.
To address these problems, Dr. Aboody and colleagues at the City of Hope have extensively characterized a clonal human NSC line (HB1.F3.CD21), that is v-myc immortalized to maintain its stem-like properties, which has demonstrated tumor tropism, chromosomal and functional stability, non-tumorigenicity, and is HLA class II negative [Aboody et al., Sci Trans Med 2013]. Clinical safety of this NSC line has been confirmed in several clinical brain tumor trials [Portnow et al., Clin Ca Res 2017]. The viral loading, release kinetics, and freeze-thaw standard operating procedures (SOPs) for transduction of the HB1.F3.CD21 NSCs with a CRAd-Survivin-pk7 (NSC.CRAd-S-pk7) are well established, and a GMP bank is currently in clinical trial as an adjunct oncolytic virotherapy for newly diagnosed glioma (NCT03072134) [Ahmed et al., JNCI 2013]. These established SOPs enable generation of additional “off-the-shelf” allogeneic clinical cell banks, providing an accelerated route to clinical translation for ovarian cancer.
In the current study, Mooney et al. demonstrate the ability of this NSC line to improve tumor-localized production of an oncolytic adenovirus in pre-clinical immunodeficient and immunocompetent models of peritoneal ovarian metastases. The viral payload used is a CRAd driven by the survivin promoter (CRAd-S-pk7). Because the survivin protein is highly expressed in ovarian cancer, but not in normal differentiated cells, viral replication occurs selectively in tumor cells. Evidence is provided supporting the hypothesis that NSCs can protect oncolytic viral cargo from neutralizing antibodies within patient ascites, and improve viral delivery and distribution to tumors in both xenograft and syngeneic tumor model systems. We demonstrated improved antitumor activity of NSC-mediated delivery vs. free CRAd-S-pk7 virus, which was further enhanced by the addition of cisplatin. Further investigations to optimize therapeutic efficacy include dose and timing of multiple treatment rounds, and the addition of checkpoint inhibitors.
Overall, these studies proof-of-concept for the translational potential of peritoneal oncoviral delivery by tumor-tropic NSCs. Used as an adjunct to chemotherapy and/or checkpoint inhibitors, this novel NSC oncolytic virotherapy strategy shows promise for improving clinical outcome in patients with advanced ovarian cancer. In addition, the selective delivery of oncolytic virus by tumor tropic cells, as well as use of a tumor-specific promotor should minimize off target toxicities, thereby improving quality of life during treatment.

Society News
ASGCT Selects 14 Recipients of Inaugural Career Development Grants, Awards Total $245,000
ASGCT is honored to have received such strong interest from its membership and congratulates this strongly-qualified group of awardees. The 2019 Career Development Grants program will begin accepting applications shortly after the 22nd ASGCT Annual Meeting, April 29 – May 2, held at the Washington Hilton in Washington, D.C.
See the Full 14-Recipient Roster
Abstract Submission Closes Next Week!
The ASGCT abstract submission deadline, Wednesday, January 16, 2019 (5 p.m. CT), is now less than one week away! Submit your abstract today for the opportunity to present your work at the 22nd Annual Meeting. As always, Associate Members who are the first and presenting author on an abstract (either oral or poster) receive free meeting registration!
Submit an Abstract Online

Public Policy
FDA Operating, Running Limited Programming During Partial Government Shutdown
The Food and Drug Administration has continued to operate at partial capacity during the partial federal government shutdown, which began on Dec.22. Without a functioning government, the agency is limited to activities:
- Necessary to address imminent threats to the safety of human life
- Funded by carryover funds, notably but not exclusively, 2018 user fees.
Though the FDA can operate on carryover funds, the agency is unable to accept new user fees during a government shutdown and those funds will start to run out within the next month without further action. FDA Commissioner Scott Gottlieb said in a Twitter thread on Jan. 5 that PDUFA funds will likely run out first and that the agency will release a more detailed funding analysis soon.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet