The Vector
Volume 8, Issue 3: March 2019
Editorial Team
Phillip Doerfler, PhD - Editor, The Vector
Melvin Rincon, MD, PhD - Associate Editor, The Vector
Edith Pfister, PhD - Junior Editor, The Vector
Inside This Issue
Leadership Message
Breaking Through
Society News
Public Policy
Industry News
Secretary's Message
Get Involved With The Annual Meeting!
Vote and Volunteer with ASGCT
 The 22nd Annual Meeting is barely more than a month away (April 29–May 2 at the Washington Hilton), but there’s still time to get involved! The Society is looking for volunteers to review posters across all of our abstract categories. More than half of all abstracts presented at the Annual Meeting is presented during one of the poster sessions during the evenings of the Annual Meeting, so this is a great opportunity to more intimately review some of this groundbreaking research.
The 22nd Annual Meeting is barely more than a month away (April 29–May 2 at the Washington Hilton), but there’s still time to get involved! The Society is looking for volunteers to review posters across all of our abstract categories. More than half of all abstracts presented at the Annual Meeting is presented during one of the poster sessions during the evenings of the Annual Meeting, so this is a great opportunity to more intimately review some of this groundbreaking research.
Members are invited to select up to three categories to review. From there, ASGCT leadership will review the entries and assign reviewers.
Volunteer on ASGCT.org
And as a leader in ASGCT, I also personally implore all members and transitional members to take a few minutes to vote in the 2019 ASGCT election. It has been my privilege to serve as the secretary for ASGCT for 3 years, and I am excited to see two terrific new candidates offering their talents to direct our program for the next three years.
This is a particularly big year for ASGCT elections as the Society will welcome three new members to the board of directors and two members to the ASGCT Executive Committee, secretary included. Members can vote in on the Members site of ASGCT.org until March 29.
Vote for the ASGCT Board of Directors
Thank you for allowing me to serve as ASGCT secretary for these past three years, and thank you for ensuring the future of our organization by actively participating in ASGCT, whether that’s through volunteering, voting, or both.
Best,
David V. Schaffer
Advertisement

Advertisement
Breaking Through
Protease Activatable Adeno-Associated Virus Vector for Gene Delivery to Damaged Heart Tissue
Caitlin M. Guenther, Mitchell J. Brun, Antonette D. Bennett, Michelle L. Ho, Weitong Chen, Banghe Zhu, Michael Lam, Momona Yamagami, Sunkuk Kwon, Nilakshee Bhattacharya, Duncan Sousa, Annicka C. Evans, Julie Voss, Eva M. Sevick-Muraca, Mavis Agbandje-McKenna, Junghae Suh
Summary by Dr. Edith Pfister
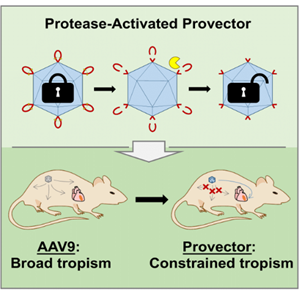 Adeno-associated virus (AAV) vectors have risen to prominence as a potential gene therapy for numerous diseases. AAV9 is particularly attractive, as it can be produced at high titers and distributes broadly in vivo. The usefulness of gene therapy in cardiac diseases has been limited by off-target effects in other tissues. An AAV variant that can deliver cargo specifically to sites of tissue damage would make cardiac gene therapy more broadly applicable.
Adeno-associated virus (AAV) vectors have risen to prominence as a potential gene therapy for numerous diseases. AAV9 is particularly attractive, as it can be produced at high titers and distributes broadly in vivo. The usefulness of gene therapy in cardiac diseases has been limited by off-target effects in other tissues. An AAV variant that can deliver cargo specifically to sites of tissue damage would make cardiac gene therapy more broadly applicable.
In previous work1 the group developed an activatable version of AAV2 by insertion of a cleavable peptide sequence close to the binding domain for the cell surface receptor, heparan sulfate proteoglycan (HSPG). In the presence of matrix metalloproteinases (MMPs), which are upregulated in the extracellular matrix at sites of cardiovascular damage, cleavage of the peptide exposes the HSPG binding domain, allowing internalization of the vector. In this report, Guenther et. al. developed an activatable AAV9-based vector with potential therapeutic applications.
The authors first attempted to insert a cleavable peptide sequence adjacent to amino acid residues important for galactose binding of AAV9. These initial variants displayed low viral titers or the inability to transduce cells, even in the presence of MMPs. Several variants were sensitive to cleavage by MMPs, but proteolysis failed to restore transduction capabilities indicating that insertion of the peptide in these positions permanently blocks galactose binding. The insertion site was then placed after the glycine residue at position 453 (G453). In this position, which is farther away from the galactose binding domain, the peptide insertion was tolerated and AAV titers approximated those of the parental capsid. Cryo-electron microscopy indicated that the structure of the new capsid (L001) resembles that of AAV9 but unlike AAV9, L001 is sensitive to cleavage by MMP-2, MMP-7 and MMP-9. Unlike AAV9, which transduces CHO-Lec2 cells without MMP exposure, L001 exhibits low levels of basal transduction of CHO-Lec2 cells. Transduction increases 5-fold after treatment with MMPs.
In mice, permanent ligation of the left anterior descending artery (LAD) serves as a model of myocardial infarction. To show in vivo activation and efficacy of the approach, the authors injected an activatable vector containing infrared fluorescent protein (iRFP) intravenously in LAD mice. Whereas AAV9 and control vectors exhibit the expected expression of iRFP throughout the heart, L001-iRFP co-localizes with sites of MMP activity. The L001 vector also exhibits decreased delivery of viral genomes to off-target tissues, and reduced neutralization of AAV9 transduction.
Various methods are available to limit transgene expression in vivo. Development of new AAV variants with favorable transduction profiles is promising and regulatory elements are frequently incorporated into the transgene cassette. Activatable AAVs provide an added layer of control, further limiting off target expression of transgenes, targeting sites of tissue damage and extending the usefulness of AAVs for gene therapy.
1. Judd J, Ho ML, Tiwari A, Gomez EJ, Dempsey C, Van Vliet K, et al. Tunable protease-activatable virus nanonodes. ACS Nano. 2014 May;8(5):4740–6.
Advertisement

Advertisement
Society News
Vote in The 2019 ASGCT Election!
The 2019 ASGCT ballot is now open in the members’ site at ASGCT.org. The ballot is available to members and transitional members after logging in with your ASGCT account. Voting is open through March 29. Members are invited to select one candidate for each of the following positions:
- Vice President
- Secretary
- At-Large Director 1
- At-Large Director 2
- At-Large Director and Membership Council Chair
2.5 Million Euro Funding Opportunity Accepting Applications
The 2020 Else Kröner Fresenius Award for Medical Research is accepting applications in the fields of gene editing and gene therapy through April 4. The biennial research will distribute 500,000 Euro directly to the recipient with an additional 2 million Euro dedicated to the awardee’s research efforts. Candidates “must be researchers who have made significant scientific contributions to the field of genome editing and gene therapy and are expected to yield groundbreaking discoveries in the near future.”
Learn more about the
Else Kröner Fresenius Award
for Medical Research
Advertisement

Advertisement
Public Policy
CMS Proposes Coverage With Evidence Development of CAR T-Cell Therapies
The Centers for Medicare & Medicaid Services (CMS) issued a proposed decision memo last month regarding the National Coverage Analysis (NCA) of CAR T-cell therapies. The memo proposes coverage with evidence development (CED), which is a paradigm whereby Medicare would cover the biologic on the condition that it is furnished with the collection of additional clinical data. ASGCT is concerned that CED may limit patient access to CAR T-cell therapies and will submit specific concerns to CMS this week. Society recommendations will include removing the requirement that patients receiving CAR T-cell therapy have relapsed or refractory cancers, to allow coverage of potential upcoming first-line indications for CAR T-cell therapies without reopening the NCA. CMS will issue the final decision memo by May 17.
Final FDA Guidance Addresses ASGCT Comments
Last month, the FDA finalized its guidance document titled “Expedited Programs for Regenerative Medicine Therapies for Serious Conditions.” ASGCT is pleased that the final document featured revisions that addressed comments the Society submitted last year. ASGCT requested FDA to clarify that gene therapies are considered to be regenerative medicine advanced therapies (RMATs) and are therefore eligible for the RMAT designation. The final guidance clarifies the criteria for gene therapies to be considered RMATs—those that “lead to a sustained effect on cells and tissues.”
Additionally, the Society requested more information on innovative trial design. The FDA added to the final document that historical controls may be considered as appropriate, and that natural history data can be used as the basis of a historical control when the control and treatment populations are adequately matched. The document also added details for “trials that compare several different investigational agents to each other and a common control.” Each practice needs to meet the biologics license application (BLA) requirements, and product manufacturing needs to meet current good manufacturing practices requirements.
ASGCT Supports Novel Payment Models
On the legislative front, ASGCT submitted comments last month to Senators Cassidy (R-LA) and Warner (D-VA) on a piece of draft legislation they have proposed. The Patient Affordability, Value, and Efficiency (PAVE) Act would remove regulatory and legal barriers to agreements that contain novel, value-based payment arrangements for medical treatments. The Society expressed support for enabling such payment arrangements for gene therapy products. Agreements that include provision of a lower price to patients and payers in instances in which a patient does not respond adequately to treatment creates a more equitable distribution of costs across patients and payers, while retaining encouragement for further innovation in gene therapy development. Additionally, ASGCT recommended legislators consider policies that allow payment over time arrangements for gene therapies, to address the potential challenge to payers of upfront costs for one-time treatments.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet