The Vector
Volume 8, Issue 6: June 2019
Editorial Team
Melvin Rincon, MD, PhD – Editor, The Vector
Edith Pfister, PhD – Associate Editor, The Vector
Inside This Issue
Leadership Message
Breaking Through
Society News
Public Policy
Industry News
Leadership's Message
ASGCT Heats Up the Summer
Up-to-date clinical trials info and a new policy-centered event keep up the post-meeting momentum.
 The summer has historically been a slow period for ASGCT, but that’s changed in recent years. As one of the newly-elected members of the Society’s board of directors, I’m proud to say ASGCT is now just as busy in June as it is in January.
The summer has historically been a slow period for ASGCT, but that’s changed in recent years. As one of the newly-elected members of the Society’s board of directors, I’m proud to say ASGCT is now just as busy in June as it is in January.
Recently, the Society launched the ASGCT Clinical Trials Finder, the only curated, interactive tracking system for gene and cell therapy clinical trials recruiting in the United States. This customizable search engine allows users to explore active clinical trials across the field based on nearly a dozen user-decided criteria.
The launch of the ASGCT Clinical Trials Finder comes at the perfect time as NIH begins phasing out their Genetic Modification Clinical Research Information System, which served a similar purpose. NIH has committed to make stakeholders aware of the resource and ASGCT will work to migrate users of the GeMCRIS system to the ASGCT service.
On behalf of ASGCT, thank you to all of the committee members, stakeholder groups, and Society members who lent their time and expertise to make this important project a reality. We're looking forward to hearing your thoughts on this new tool. Tell us about your experience by emailing info@asgct.org.
 ASGCT is also hard at work on a second, mid-cycle event opposite the ASGCT Annual Meeting. The ASGCT Policy Summit (November 4 – 6 at The Westin in Washington, D.C.) will gather scientific, regulatory, and bioethical communities for three days of discussion focusing on global regulation, payment policies, and the ethics of germline gene editing.
ASGCT is also hard at work on a second, mid-cycle event opposite the ASGCT Annual Meeting. The ASGCT Policy Summit (November 4 – 6 at The Westin in Washington, D.C.) will gather scientific, regulatory, and bioethical communities for three days of discussion focusing on global regulation, payment policies, and the ethics of germline gene editing.
Each day covers one of the aforementioned topics and can be registered for individually to provide stakeholders flexibility. The schedule breakdown is as follows:
- November 4: Global Regulatory Issues in Gene Therapy Development
- November 5: Perspectives on Payment Policies
- November 6: Scientific, Ethical, and Societal Issues in Germline Gene Editing
Additional programming and speaker information will be released on ASGCT.org prior to the opening of ASGCT Policy Summit registration on July 8.
I’m looking forward to sharing all of the great initiatives we’re undertaking at ASGCT and hope to see many of you at the summit this November.
Sincerely,
Maritza McIntyre, Ph.D.
Advertisement

Advertisement
Banner space is available in the July and August editions of The Vector. Learn how you can target an engaged audience with ASGCT.
Breaking Through
A Comprehensive Single Cell Transcriptional Landscape of Human Hematopoietic Progenitors
Danilo Pellin, Mariana Loperfido, Cristina Baricordi, Samuel L. Wolock, Annita Montepeloso, Olga K. Weinberg, Alessandra Biffi, Allon M. Klein, and Luca Biasco
DOI: https://doi.org/10.1038/s41467-019-10291-0
Summary by Luca Biasco, Danilo Pellin and Mariana LoperfidoASGCT 22nd Annual Meeting Recap
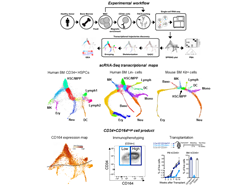 It is established knowledge that human hematopoietic stem/progenitor cells (HSPCs) constitute a heterogeneous population residing in the bone marrow (BM) endowed with the role of generating and maintaining the diverse pool of blood cells lifelong. However, despite recent advances, the hierarchical relationships linking the different hematopoietic progenitors and the nature of early hematopoietic cell fate choices have remained unresolved and highly contentious. The recent advent of single-cell RNA sequencing (scRNA-Seq) has created an opportunity to improve our understanding of human hematopoiesis through the study of transcriptional single-cell states. Still, initial use of this technology in humans generated often unclear and conflicting observations, leading many researchers to progressively abandon the idea of a structured hematopoietic tree. In this study, making use of a fine-tuned partitioning method to stratify bone marrow (BM) lineage negative (Lin-) cells combined with high-resolution InDrops scRNA-Seq, we instead revealed that hematopoietic differentiation is a hierarchically-structured transcriptional landscape and we provided a detailed snapshot of the early stepwise cell fate choices governing human hematopoiesis.
It is established knowledge that human hematopoietic stem/progenitor cells (HSPCs) constitute a heterogeneous population residing in the bone marrow (BM) endowed with the role of generating and maintaining the diverse pool of blood cells lifelong. However, despite recent advances, the hierarchical relationships linking the different hematopoietic progenitors and the nature of early hematopoietic cell fate choices have remained unresolved and highly contentious. The recent advent of single-cell RNA sequencing (scRNA-Seq) has created an opportunity to improve our understanding of human hematopoiesis through the study of transcriptional single-cell states. Still, initial use of this technology in humans generated often unclear and conflicting observations, leading many researchers to progressively abandon the idea of a structured hematopoietic tree. In this study, making use of a fine-tuned partitioning method to stratify bone marrow (BM) lineage negative (Lin-) cells combined with high-resolution InDrops scRNA-Seq, we instead revealed that hematopoietic differentiation is a hierarchically-structured transcriptional landscape and we provided a detailed snapshot of the early stepwise cell fate choices governing human hematopoiesis.
In the present work we:
- Clarified the heterogeneous nature of the major seven HSPC subsets into the context of a newly generated high-resolution BM Lin- scRNA-Seq map
- Investigated the expression of transcription factors along differentiation trajectories and branching points (valuable for in vitro reprogramming efforts)
- Provided a detailed comparison of the expression of gene orthologues in human vs mouse hematopoiesis (valuable for the modeling of blood cell disorders in the mouse)
- Performed a full computational, immunophenotypic and functional validation of the origin of the basophil branch with confirmation of its proximity to the megakaryocyte-erythroid progenitors and with the proposal of the CD135 marker as discriminator for progenitor cells with basophil potential (Lin-CD34+CD135- cell population)
- Identified CD164 as a reliable marker for the selection of a CD34+ cell fraction enriched in primitive progenitors (CD34+CD164high cell population) in different cells sources and, in comparison, with CD34+CD90+ cell fraction
- Validated the repopulation capacity of CD34+CD164high cell population in vivo which constitutes a novel self-sufficient cell product potentially suitable for transplantation and gene therapy
We started by fractionating and analyzing at single cell level the BM CD34+ compartment, which conventionally contains cell populations at the initial stage of hematopoietic differentiation. The resulting map of 6011 single cell transcriptomes showed a hierarchical, tree-like continuum of states with branches edges expressing recognizable transcriptional signatures of lineage commitment before the expression of final maturation markers. We could observe that the earliest fate split separates erythroid-megakaryocyte progenitors from lymphoid-myeloid progenitors, which then separate further into lymphoid, dendritic cell and granulocytic progenitors. We noted, however, that branches towards basophils and monocytes commitment were missing. Indeed, an overlooked aspect of earlier works is that many cells negative for mature lineage markers in human BM are CD34low/-. These cells have a much lower probability of being captured upon magnetic beads separation or conventional FACS-sorting for CD34+ cells, and this has prevented collecting information on HSPCs transitional states at which the CD34 expression is rapidly down-regulated.
Therefore, differently from previous studies, we here also extended our analysis outside the CD34+ compartment to the whole Lin- cell fraction of the human BM. In doing so we made use of a cell surface molecule, CD164, which we identified from the initial data set as a gene whose expression displayed the most-pronounced difference in early vs late progenitors. On the basis of this marker, we designed a novel graded FACS-sorting strategy that corrects for expansion of cells as they differentiated and thus allows examining early states alongside later ones that comprise the vast majority of Lin- progenitors. The result of this analysis was the generation of a high-resolution transcriptome landscape of 15,401 cells inside the whole Lin- fraction of the human BM. Through this map we could unveil the complete underlying structure of early adult human hematopoiesis, including an extended coverage of transcriptional transitions leading to erythroid differentiation, and we could recover key missing branch-points into basophils and monocytes branching. Our study presents detailed information on the expression of transcription factors and other gene classes at branching points and along differentiation trajectories. Notably, we showed that the topology of the human landscape was remarkably similar to what previously reported in the mouse BM Kit+ transcriptome map, so much that we could identify a number of gene orthologues expressed along the erythroid differentiation whose expression dynamics seems to be evolutionarily conserved between these two species. A most-notable result emerging from the exploration of our BM Lin- map was the identification of a branch toward cells carrying a transcriptional profile of early basophils specification. Strikingly, this class of basophils progenitors (BaP) was found to associate with erythroid and megakaryocyte fates and not with granulocytes precursors. In our work, we validated the origin of this branch in silico and in vitro, identifying the Lin-CD34+CD135- fraction as containing primitive progenitors already primed with megakaryo-erythro-basophil potential.
To test the translational potential of the information contained in our study, we asked whether we could take advantage of the surface marker CD164, previously identified through our sc-RNAseq maps, to select a new subset of CD34+ cells potentially suitable for transplantation and gene therapy. Through cytometric analysis, we validated the CD34+CD164high as a cell fraction representing 60% of CD34+ cells in the BM which is highly enriched in early megakaryo-erythroid and myeloid progenitors, while it is almost entirely depleted of pre-B/NK and Lin+ cells. We tested this population phenotypically and functionally in vitro vs the classical CD34+CD38- and the CD34+CD90+CD45RA- cell fractions and in vivo a in xenotransplantation model. Based on the evidences collected in our work, we could show that the use of CD164 allows for a higher discriminatory potential in identifying a population with multilineage potential and we discovered that the CD34+CD164high population might constitute a novel, efficacious and self-sufficient cell product for transplantation and gene therapy.
The following key features would make such selection strategy a promising approach for clinical translation:
- Automatically excludes pre-B precursors, providing a system worth exploring for the potential exclusion of residual leukemic cells with early B-cell commitment in transplantation products for B-cell leukemia
- Allows isolating a self-sufficient product capable of sustaining both early and late phases of hematopoietic reconstitution, an advantage over other currently proposed selection strategies that, in most cases, require co-transplantation of other committed precursors to sustain early myelopoiesis
- Could reduce of about a half the number of target cells needed for genetic engineering in clinical gene therapy, in turn reducing of 50% the costs for manufacturing of gene transfer/gene editing platforms
- Allows at the same time the infusion of a sizeable portion of CD34+ cells, making clinical actors more inclined on accommodating its use as improvement of long-established clinical protocols than what would be the case for other selection strategies based on smaller and less defined CD34+ cell fractions
- Combines only two surface markers (CD34 and CD164), thus allowing the design of strategies based on magnetic beads selection, a more suitable and scalable approach for the clinical arena than current proposals based on FACS-sorting.
Overall, our study provides a detailed view of the early hematopoietic cell state hierarchy in humans and a valuable resource for the study of the biology of human hematopoiesis and for the clinical exploitation of human HSPCs.
Society News
ASGCT Launches Clinical Trials Finder
Follow gene and cell therapies through the developmental pipeline using the new ASGCT Clinical Trials Finder.
The ASGCT Clinical Trials Finder is a customizable search engine for active and recruiting trials in gene and cell therapy throughout the United States. Data curated daily from ClinicalTrials.gov ensures the most up-to-date and applicable listings anywhere in the field, displayed in a reader-friendly format.
ASGCT members who volunteered to assist in the development of the ASGCT Clinical Trials Finder reviewed the relevance of the parameters with which the system pulls in data and created a list of important terms to best identify and categorize ongoing clinical trials.

ASGCT to Award $300,000 to Members in 2019
ASGCT will award a $50,000 Career Development Award in each of the following six broad topics. The purpose of these awards is to support independent transformative pilot studies in gene and cell therapy by ASGCT members, particularly those ideas that would be challenging to fund with normal funding mechanisms. ASGCT is interested in helping applicants generate their preliminary data to use in larger proposals (NIH K awards, first-submission R-level funding, etc.) to gain independence. Learn more about the eligibility requirements, application materials, and key deadlines in the Career Development Award Overview and Benefits.
View Slides From Sold Out Pre-Meeting Workshops
ASGCT's record breaking workshop attendance meant that all four workshops sold out weeks before the 22nd Annual Meeting. Due to increased interest in the workshop materials, presenter-approved slides for each workshop can be found on asgct.org.
Annual Meeting Award Recipients
Congratulations to the winners of the 2019 Excellence in Research, Outstanding Poster, and Travel awards presented at the 22nd Annual Meeting. A full list of award honorees is available on the 22nd Annual Meeting website.
Public Policy
Sickle Cell Disease Briefing on the Hill
ASGCT and Senators Tim Scott and Cory Booker are co-hosting a briefing on sickle cell disease (SCD), in collaboration with other stakeholder organizations on June 18 at 2:30 – 3:30 p.m. in Washington, DC. The briefing, scheduled to take place in the Dirksen Senate Office Building, Room G50, will feature leading experts in the field discussing progress in gene therapy and gene editing for SCD, as well as the policy implications surrounding that progress. Policy topics that will be addressed during the briefing are robust NIH funding; reauthorization of the newborn screening program; novel payment arrangements for FDA-approved gene therapies, in preparation for future potential approvals for SCD; and funding of the SCD prevention and treatment program, and implementation of new surveillance and screening authorities passed in 2018 legislation. Those interested in attending should RSVP to Betsy Foss-Campbell, Director of Policy and Advocacy, at bfoss@asgct.org by June 12.
Outside Witness Testimony Calls for Robust NIH Funding
Last week ASGCT submitted outside witness testimony to the Senate Labor, Health and Human Services, Education, and Related Agencies Appropriations subcommittee regarding NIH funding. In the testimony, the Society requested robust funding to the NIH to support continued advancements in the field of gene and cell therapy. The letter expressed the significance of NIH research funding to gene and cell therapy, which has played a significant role in advancements of these therapies for diseases such as sickle cell disease. The Society also highlighted the need for additional funding in the field of gene and cell therapy research to spur innovation in the field that will lead to the development of additional transformative therapies. The Society called attention to the fact that federal funding to the NIH still lags behind the level in 2003, when adjusted for biomedical research inflation. ASGCT had two main requests for Senate appropriators through this letter. The Society requested an increase of at least $2 billion to the NIH general fund for FY2020, as the House has proposed, and appropriation of the $8 million authorized by the Cures Act for FY2020 for the Regenerative Medicine Innovation Project.
Industry News
Interested in advertising in The Vector?
View the Rate Sheet