The Vector
Volume 7, Issue 3: May 2018
Editorial Team
Guohua Yi, PhD - Editor, The Vector
Phillip Doerfler, PhD - Associate Editor, The Vector
Melvin Rincon, MD, PhD - Junior Editor, The Vector
Inside This Issue
President's Message
Breaking Through
Society News
Public Policy
Industry News

President's Message
 Dear Colleagues,
Dear Colleagues,
We have had a long pleasant spring in Houston this year and now that May is here spring has also reached Chicago in time for the 21st Annual Meeting of ASGCT which is now only two weeks away. Among the many events planned, I am delighted to welcome the invited keynote speakers to our four general sessions. This year, the Society is honoring Dr. Jean Bennett, who is receiving the Outstanding Achievement Award, and Drs. Luca Biasco and Luk Vandenberghe who are receiving the Outstanding New Investigator Awards. Each will be presenting a lecture highlighting their scientific achievements. Additionally, we are honoring Fondazione Telethon and its General Director Francesca Pasinelli, who will be accepting in person, with the Sonia Skarlatos Public Service Award. If you have not yet registered for the Annual Meeting, you can still do so online through May 10 or in person at the Chicago Hilton.
I am also looking forward to keynote lectures from Drs. Kathy High and Nick Restifo. Dr. High will deliver the George Stamatoyannopoulos lecture on Friday May 18th. Dr. High began her career studying the molecular basis of blood coagulation and the development of novel therapeutics for the treatment of bleeding disorders including gene therapy strategies. Her work on gene therapy for hemophilia led to a series of studies that characterized the human immune response to AAV vectors in a variety of target tissues and has now progressed to clinical translation of potential genetic therapies for multiple inherited disorders. Dr. Nick Restifo will be delivering the Presidential Symposium on Thursday May 17th. Dr. Restifo’s research focuses on enhancing T cell-based immunotherapies and he has made broad contributions to the field of cancer immunity including identification of myeloid derived cell subsets that suppress anti-tumor T-cell responses and discovery of a novel subset of long lived human T memory stem cells in humans.
I wish to thank you all for voting in the recent Board of Directors election. The Society has elected four new members to the Board of Directors, including a new incoming vice president. Each elected member will serve a three-year term, including the incoming vice president who will enter the leadership succession line. Stephen Russell, MD, PhD will serve a yearlong term as vice president before serving as ASGCT's president-elect and, finally, president through the 2021 annual meeting. I am pleased to welcome Richard Morgan, PhD as the Translational and Clinical Development Representative, Jennifer Adair, PhD and Stephen Hart, PhD as At-Large Directors. All four of the newly-elected members will begin their terms at the conclusion of 21st Annual Meeting.
I look forward to seeing you all in Chicago in two short weeks!

Breaking Through
Du X, Cai Q, West MB, Youm I, Huang X, Li W, Cheng W, Nakmali D, Ewert DL, Kopke RD, Regeneration of Cochlear Hair Cells and Hearing Recovery through Hes1 Modulation with siRNA Nanoparticles in Adult Guinea Pigs, Molecular Therapy (2018), doi: 10.1016/j.ymthe.2018.03.004
Summary written by Matthew B. West and Xiaoping Du
Sensorineural hearing loss (SNHL) is a highly prevalent condition in the human population, impacting the lives of more than 600 million people worldwide. SNHL represents a chronic condition, as the cumulative loss of auditory sensory hair cells (HCs) in the cochlea, resulting from acoustic traumas, ototoxic insults, or age-related attrition, is irreversible in mammals, leading to permanent deafness. However, previous studies conducted in our laboratory and others have demonstrated that targeted, transient inhibition of key homeostatic signaling pathways can lead to de novo HC production through a process called trans-differentiation that involves reprogramming of resident cochlear supporting cells to adopt a terminal hair cell fate.
A gene therapy approach based on synthetic small interfering RNA (siRNA) delivery into target cells to elicit RNA interference (RNAi) of a specific messenger RNA represents a compelling method to achieve the desired degree of precision in this regenerative process. Using this therapeutic strategy, we previously demonstrated that transient transfection of siRNAs that target the Notch pathway gene, hairy and enhancer of split 1 (Hes1), could induce HC regeneration within organotypic cultures of neonatal ototoxin-ablated murine cochleae (Du et al., 2013). Furthermore, encapsulation of these siRNA molecules within biocompatible poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) retained their regenerative properties, allowing for enhanced tissue compatibility and stability of the therapeutic payload (Du et al., 2013).
In our most recent published study, we sought to determine if these regenerative properties could be translated to a live animal model and, if so, would the inducible degree of HC restoration confer functional recovery (Du et al., 2018). Using adult guinea pigs exposed to deafening noise, we infused PLGA NPs containing a fixed dose of either scrambled (sham) or Hes1 siRNA (siHes1) into the cochlear labyrinth over a period of 24 hours, using micro-osmotic pumps. To minimize the possibility for potential cytoprotective effects, we waited to initiate treatment after a 72-hour delay post-trauma. Serial auditory brainstem response (ABR) recordings, an objective measure of hearing function, were performed 24 hours after the noise trauma and at subsequent intervals of 3, 6, and 9 weeks post-treatment for both the sham and siHes1 NP cohorts. Complementary histological evaluations were carried out after the terminal 9-week ABR recording to assess the treatment-specific effects on HC restoration post-deafening across the aggregate length of the cochlear spiral.
The results from this study proved to be very emboldening. At the histological level, the noise injury resulted in pervasive HC loss, such that only 46.7% and 18.3% of the original population of inner (sound-transducing) and outer (sound-amplifying) HCs, respectively, remained in noise-deafened guinea pigs treated with sham NPs at nine-weeks post-injury. In comparison, noise-injured cochleae treated with PLGA NPs loaded with siHes1 biomolecules presented at this same time point with inner HC (IHC) and outer HC (OHC) numbers that approached 75.1% and 55.9% of the original populations observed in uninjured controls, representing a significant degree of HC regeneration across the tonotopic breadth of these cochleae. In siHes1 NP-treated ears, the vast majority of IHCs and OHCs possessed stereocilia and were organized in cytoarchitecturally-appropriate positions within the sensory epithelial region, although ectopic IHCs and OHCs were also uniquely observed in these cochleae. Tissues harvested at earlier timepoints post-treatment revealed evidence of both morphologically- and immune-histologically-immature IHCs and OHCs, consistent with ongoing maturation and de novo HC production in this treatment group. Coincident with this robust HC regenerative response, statistically- and clinically-significant (i.e. >10dB) functional recovery of ABR threshold sensitivity was consistently measured among animals in the active treatment group at each of the test frequencies (2, 4, 8, 16 kHz) evaluated in this study and persisted out to the terminal nine-week timepoint. No such functional recovery was measured in sham NP-treated ears. Taken together, these observations indicate that siHes1 NP treatment is capable of inducing widespread and sustainable HC regeneration and functional recovery in adult mammalian cochleae.
These findings provide strong preclinical support for this technology as a potential therapeutic for treating SNHL. Future studies will focus on refining compositional and dosing parameters for the therapeutic formulation, as well as simplification of the drug administration route, using a more facile middle ear delivery approach. Studies aimed at evaluating siHes1 NP-dependent functional recovery in animals with long-standing hearing loss are also anticipated.
Graphical Abstract
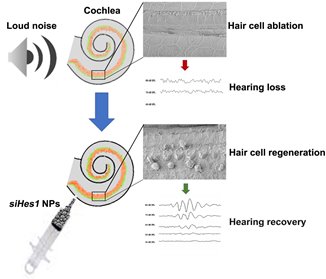
Localized infusion of siHes1 nanoparticles resulted in treatment-specific regeneration of auditory hair cells and functional recovery in adult, noise-deafened guinea pigs.

Inner ear direct neuronal reprogramming for the restoration of hearing
Noda, T., Meas, S. J., Nogami, J., Amemiya, Y., Uchi, R., Ohkawa, Y., et al. (2018). Direct Reprogramming of Spiral Ganglion Non-neuronal Cells into Neurons: Toward Ameliorating Sensorineural Hearing Loss by Gene Therapy. Frontiers in Cell and Developmental Biology, 6, 16. http://doi.org/10.3389/fcell.2018.00016
Summary written by Steven J. Meas and Alain Dabdoub
Current interventions for the treatment of hearing loss depend exclusively on devices, such as hearing aids and cochlear implants. These devices require a high level of concentration and extensive training with certified audiologists. Additionally, users often complain of difficulty listening in noisy settings and have hardship attempting to resolve complex sounds such as music (Kujawa and Liberman, 2015; McDermott, 2004). Despite these drawbacks, these instruments have had a profound impact on individuals who experience hearing loss. These devices rely on intact biological circuits in the inner ear, including the primary auditory neurons, which form the connection between the peripheral auditory system and the brainstem. Hence, assistive hearing devices are not compatible with individuals who have lost their auditory neurons due to degeneration, disease or aging.
On a population level, debilitating hearing loss, as defined by the World Health Organization, impacts over 5% of the world’s population. This number includes the 0.3% of infants born with congenital birth defects and the 50% of adults over the age of 70 with hearing damaged through the natural processes of aging (Kral and O'Donoghue, 2010; Sprinzl and Riechelmann, 2010). Worldwide estimates of hearing loss are additionally projected to double within the next 30 years due to a globally aging population. Hence, there is an increasingly evident impetus to develop new strategies to improve the quality of lives for individuals experiencing hearing impairment now and in the future (Meas et al., 2018).
In our recently published studies, we propose direct reprogramming as a biological intervention to restore hearing in individuals who have lost their primary auditory neurons. By employing cellular and transcriptomic based techniques, we have shown that cells located in the spiral ganglion, the site of primary auditory neuron cell soma, are excellent candidates for direct reprogramming into induced neurons for the potential reconstruction of auditory circuits and the amelioration of hearing loss.
We were able to reprogram neonatal spiral ganglion non-neuronal cells into neuron-like cells. We ectopically expressed two neurogenic transcription factors; Ascl1, a potent neurogenic pioneer factor, and NeuroD1, an auditory neuron differentiation factor. Ascl1 is capable of manipulating chromatic structure to access gene targets and can convert even distant lineages into induced neurons (Chanda et al., 2014). NeuroD1, on the other hand, is needed for differentiation of endogenous auditory neurons (Kim et al., 2001). We hypothesized that the combination of the two would yield induced neurons that were closer in identity to endogenous auditory neurons. Both constructs included a Ds-Red reporter to confirm transfected cells. After seven days of culturing, spiral ganglion non-neuronal cells developed into a neuron-like morphology with extensions and expressed markers of endogenous auditory neurons; βIII-tubulin, microtubule-associated protein 2 (MAP2), as well as vesicular glutamate transporter 1 (Vglut1) and prospero homeobox protein 1 (Prox1). These induced neurons were also capable of developing extensions towards co-cultured explants of endogenous primary auditory neuron targets; the mechanosensory hair cells of the inner ear and the cochlear nucleus neurons of the brainstem.
We established a transcriptomic pipeline to analyze these induced cells in greater detail and observe changes in gene expression patterns seven days after reprogramming. We harvested spiral ganglion cells from one-day old Tau-EGFP mice and performed fluorescence-activated cell sorting. Cells raised from this transgenic mouse line express EGFP at the Tau locus, which is a neuronal specific marker. Hence, cells could be collected into two fractions; EGFP+, which included the endogenous primary auditory neurons (PANs), and EGFP-, which included the spiral ganglion non-neuronal cells. RNA was directly extracted from the EGFP+ fraction (PAN), whereas the EGFP- fraction was plated onto dishes for culturing and transfected with the neurogenic transcription factors Ascl1-DsRed and NeuroD1-DsRed or a Control-DsRed vector. Transfected cells were maintained in culture for seven days and sorted based on EGFP and DsRed fluorescence intensity. Since cells originated from a Tau-EGFP background, spiral ganglion non-neuronal cells that were initially EGFP- began to upregulate the Tau locus and express EGFP as they were reprogrammed into induced neurons. This way, a pure population of induced neurons (iN) could be collected. Similarly, a pure population of cells transfected with Control-DsRed were collected, but they did not express EGFP (VC). RNA was extracted from each population and samples were then processed for next-generation RNA sequencing.
In total, 14,000 genes were detected from each of the three groups (PAN, iN and VC), offering insight into the changing expression profile of spiral ganglion non-neuronal cells upon ectopic overexpression of neurogenic transcription factors. The three groups formed discrete clusters on a 2-dimensional principal component analysis plot. We profiled gene expression based on a modified list of genes known to be expressed in auditory neurons (Treutlein et al., 2016). Both PANs and iNs highly expressed neuronal markers (e.g. Tubb3, MAP2, Dcx,); genes for synaptic proteins (e.g. Snap25, Stmn3); genes for ion channels (e.g. Kcnk3); and Prox1. However, the iN group failed to express some genes observed in PANs (e.g. Kcnk1, Kcnk9, Gata3). Although the iN group continued to express some markers observed in the VC group (e.g. glial markers - Sox2, Sox10, Ngfr; mesenchymal marker - Vim), they also had downregulated genes characteristic of the VC group (e.g. glial marker – Nes, mesenchymal marker – Tnc). We performed gene ontology analysis to parse out which pathways were being activated in the iN group compared to the VC group based on the 4,091 genes that were differentially expressed two-fold or more between the two groups. Of the top 30 biological processes, the majority of events were highly related to neurogenic processes such as neurogenesis, axonogenesis, and dendrite development. This indicated that transcriptomic profiles were transitioning from a spiral ganglion non-neuronal cell phenotype towards an induced neuron phenotype upon ectopic expression of neurogenic transcription factors.
This study highlights the potential for reprogramming cells from the spiral ganglion into induced neurons. After only seven days, cells were transformed into those displaying neuron-like morphologies, protein expression and transcriptomic profiles. This work lays the foundation for reprogramming neurons within the inner ear and underscores the importance of direct reprogramming and gene therapy for the restoration of hearing.
References
Chanda, S., Ang, C. E., Davila, J., Pak, C., Mall, M., Lee, Q. Y., et al. (2014). Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Reports 3, 282–296. doi:10.1016/j.stemcr.2014.05.020.
Kim, W. Y., Fritzsch, B., Serls, A., Bakel, L. A., Huang, E. J., Reichardt, L. F., et al. (2001). NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 128, 417–426.
Kral, A., and O'Donoghue, G. M. (2010). Profound deafness in childhood. N Engl J Med 363, 1438–1450. doi:10.1056/NEJMra0911225.
Kujawa, S. G., and Liberman, M. C. (2015). Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research 330, 191–199. doi:10.1016/j.heares.2015.02.009.
McDermott, H. J. (2004). Music perception with cochlear implants: a review. Trends Amplif 8, 49–82. doi: 10.1177/108471380400800203
Meas, S. J., Zhang, C.-L., and Dabdoub, A. (2018). Reprogramming glia into neurons in the peripheral auditory system as a solution for sensorineural hearing loss: lessons from the central nervous system. Front Mol Neurosci 11. doi:10.3389/fnmol.2018.00077.
Noda, T., Meas, S. J., Nogami, J., Amemiya, Y., Uchi, R., Ohkawa, Y., et al. (2018). Direct Reprogramming of Spiral Ganglion Non-neuronal Cells into Neurons: Toward Ameliorating Sensorineural Hearing Loss by Gene Therapy. Front. Cell Dev. Biol. 6, 16. doi:10.3389/fcell.2018.00016.
Sprinzl, G. M., and Riechelmann, H. (2010). Current trends in treating hearing loss in elderly people: a review of the technology and treatment options - a mini-review. Gerontology 56, 351–358. doi:10.1159/000275062.
Treutlein, B., Lee, Q. Y., Camp, J. G., Mall, M., Koh, W., Shariati, S. A. M., et al. (2016). Dissecting direct reprogramming from fibroblast to neuron using single-cell RNA-seq. Nature 534, 391–395. doi:10.1038/nature18323.

Society News
All of the new research to be presented at the 21st Annual Meeting is now online!
ASGCT is excited and honored to lift the curtain on the incredible collection of new abstracts that will be presented at the 21st Annual Meeting, May 16-19 at the Chicago Hilton.
Now is the time to finalize your schedules with the full information on scientific presentations now available. To make that even easier, you can use the newly-release ASGCT app. Read the research, build your schedule, find other attendees, and schedule meetings through the app, available on both the Apple App Store and Google Play.

ASGCT proudly announces the newest members of the Board of Directors

Vice President
Stephen Russell, MD, PhD
Mayo Clinic

Translational and Clinical Development Representative
Richard Morgan, PhD
bluebird bio

At-Large Director
Jennifer Adair, PhD
Fred Hutchinson Cancer Research Center

At-Large Director
Stephen Hart, PhD
UCL Great Ormond Street Institute of Child Health
Employment Center
The Annual Meeting Employment Center is a resource for employers to connect with applicants on specific open positions before and during the ASGCT 21st Annual Meeting. Employers who register for the Employment Center may post open positions on the ASGCT Job Board online, view resumes of applicants who are participating in the Employment Center, and utilize a table in the Annual Meeting Employment Center to conduct in-person interviews with applicants. Applicants can apply for open positions online through the ASGCT Job Board and meet with potential employers on-site at the 21st Annual Meeting. Interested employers and applicants must register for the Annual Meeting Employment Center in order to participate in the on-site Employment Center. Visit the website for more information about the Employment Center.

Evening Reception at the Chicago Field Museum
The Evening Reception will take place at the Field Museum on Friday, May 18, 2018 from 8 - 11 pm. Explore the museum exhibits while enjoying music from the 12-piece band. Cost for reception ticket is $25. Transportation will be provided. Space is limited so sign up soon!
Public Policy
ASGCT to Advocate for Needs of the Field at Upcoming Events
Representatives of ASGCT will promote the interests of the gene and cell therapy fields in congressional meetings at a Legislative Fly-In on May 23, presented by the Alliance for Regenerative Medicine (ARM) in collaboration with ASGCT. The event provides the opportunity to elevate the voice of ASGCT with policymakers in support of issues such as robust funding for biomedical research and the FDA, as well as measures to enhance patient access to approved therapies.
In addition, ASGCT will provide a congressional briefing for the Medical Technology Caucus on July 16 on the topic of Gene Editing Technologies: Potential and Policy Implications. The Medical Technology Caucus is a group of 40 members of the House of Representatives that aims to increase congressional awareness of the issues related to medical technology and technological improvements in health care. Educating policymakers about gene and cell therapies can facilitate accurate understanding and informed decision making.
ASGCT to Issue White Paper on Value
The Society will issue a white paper this month on the value of gene therapy. The paper will inform health care payers, policymakers, and other stakeholders about the unique value of gene therapy and the resulting need to maximize patient access to approved therapies. Philip Reilly, MD, JD, from the ASGCT Government Relations Committee, will share highlights from the paper at the Commercialization Workshop on May 15, a workshop preceding the 21st Annual Meeting.
Industry News